Abstract
Flavokawain A (FKA), a major chalcone in the Kava plant, has recently demonstrated promising anti-cancer activities. A systematic evaluation of FKA's safety profile has not been reported before. In this study, male FVB/N mice were fed with an AIN-76A diet or AIN-76A diet supplemented with 0.6% (6 g/kg food) FKA or 0.6% commercial kava root extract (KRE) for three weeks. Dietary feeding of FKA did not affect food consumption and body weight. Histopathological examination of liver, kidney, colon, lung, heart, spleen, and thymus revealed no signs of FKA-induced toxicity. Biochemical serum analysis and histological examination confirmed normal organ function in FKA-treated mice. The cytotoxicity profile showed FKA had minimal side effects on bone marrow and small intestinal epithelial cells compared with Adriamycin. In addition, oral feeding of FKA increased activities of both glutathione S-transferase and quinone reductase in the liver, lung, prostate and bladder tissues of mice. In comparison, dietary feeding of 0.6% KRE increased liver/body weight ratio and decreased spleen, thymus, and testis/body weight ratios, as well as induced nodular proliferation in liver tissues. Therefore, dietary feeding FKA showed no adverse effects on major organ function and homeostasis in mice, suggesting the potential of FKA for chemoprevention study of human cancers.
Keywords: Flavokawain A, Hepatotoxicity, Kava
1. Background
According to the GLOBOCAN08 [1], Cancer Incidence and Mortality Worldwide 2008 report, age-standardized incidences of cancer, including lung and bladder cancers, in three kava drinking pacific countries (Fiji, Vanutu and Samoa) were markedly lower than those in their neighbor countries, such as Australia and New Zealand, despite of the higher percentages of smokers in their populations (up to 58.3% of man in Samoa smoke). Steiner [2] reported that the age-standardized cancer incidence for the three highest kava-drinking countries (Vanuatu, Fiji, and Western Samoa) was one fourth or one third that of non-kava-drinking countries, such as New Zealand and the United States, and non-kava-drinking Polynesians (Maoris). Uniquely, in these three kava drinking countries more men drink kava and smoke than do women, yet there is a lower incidence of cancer for men than for women. These reports have prompted us and others [3], [4], [5], [6], [7], [8], [9] to investigate the potential benefits of kava root extracts and its active components for cancer prevention.
Kava (Piper methysticum Forst) is a perennial plant indigenous to the South Pacific Islands. A water infusion of Kava roots has been safely used as a traditional beverage with relaxant effects on a daily basis in the Pacific Islands for thousands of years [10]. Recently, we and others have demonstrated that kava root extracts have potent anti-cancer and anti-carcinogenic activity in animal experiments [3], [4], [5], [11], [12]. However, there were rare reported cases of hepatotoxicity (0.25/1,000,000) linked to the use of commercial kava root extracts (from organic solvent extraction) in Western countries, which is lower than the rate of hepatic adverse effects (0.90–2.12 cases/1,000,000) for many daily-use drugs (e.g. anxiolytic benzodiazepines) [13]. To address the concern for kava hepatotoxicity and to develop a well-characterized chemopreventive candidate with minimized adverse side effects, we and others have focused on identifying non-toxic and pure kava components for cancer prevention [3], [4], [5], [6], [7], [8], [9], [14], [15], [16]. In our initial screening for anti-cancer agents from kava extracts, we found that flavokawains (flavokawain A, B and C) were the most potent agents among about 40 chemicals from kava extracts to induce apoptosis in cancer cell lines [9].
Several kava components, including kavalactones, pipermethystine, flavokawain B and contaminant hepatotoxins, have been claimed by different studies to be responsible for the reported kava hepatotoxicity [17], [18], [19], [20]. However, convincing evidence regarding kava hepatotoxicity is still lacking. Flavokawain A is a predominant chalcone, constituting up to 0.46% of kava extracts [21]. The chemical structure of flavokawain A is different from flavokawain B with an additional methoxy at position 4 [9]. Recent studies have shown that flavokawain A preferably inhibited the growth of different cancer cell lines with minimal effect on the growth of liver cell lines (i.e. L-02 and HepG2) up to 100 μM [8], [22]. In addition, flavokawain A exhibited in vivo anti-tumor activity in a bladder cancer xenograft model and in the UPII-SV40T transgenic bladder cancer mouse model [9], [23]. Therefore, flavokawain A appears to be a promising chemopreventive agent in kava extracts. To address concerns with respect to the potential hepatotoxicity of flavokawain A as a kava component, we proposed to examine possible adverse effects of a high dose flavokawain A (about 960 mg/kg body weight per day) on liver function and homoeostasis. Drinking 1–4 g of kavalactones per day (about 60 mg/kg body weight) has been considered as regular consumption of traditional kava [24]. Based on a reported method [25] a human equivalent dose of KRE used in the animal experiment here was estimated to be about 78 mg/kg body weight human dose. Compared to kava root extracts, mice fed with a high dose of flavokawain A for three weeks did not affect food consumption and body weight, as well as exhibits no adverse effects on major organ function and homoeostasis. In addition, flavokawain A induces phase II enzyme activity in different mouse tissues.
2. Materials and methods
2.1. Reagents
Flavokawain A (FKA) was purchased from sigma (St. Louis, MO). Kava root extract (KRE) at a concentration of 150 mg/ml kavalactones in 50% ethanol was obtained from Gaia Herbs (Brevard, NC). Glutathione S-Transferase (GST) Assay Kit was purchased from Cayman Chemical Inc. (Ann Arbor, MI). 2,6-Dichlorophenol-indophenol, β-glucuronidase, and sulfase were obtained from Sigma–Aldrich (St. Louis, MO). Human small intestinal cell line FHS was purchased from American Type Culture Collection (ATCC) and maintained in Hybri-Care medium (ATCC, Manassas, VA). The ATCC ensures authenticity of these human cell lines using short tandem repeat (STR) analyses. All the other reagents or solvents used were commercially available and of reagent grade.
2.2. Animal treatment with FKA or KRE for studying toxicity
For toxicity study, male FVB/N mice at 6 weeks of age were housed three per cage at 24 ± 2 °C and 50 ± 10% relative humidity and subjected to a 12 h light/12 h dark cycle. Mice were randomly divided into four groups (6 mice per group), and Diets were commercially prepared by Dyets (Bethlehem, PA). The use of male FVB/N here will facilitate our planned work to test the chemoprevention efficacy of dietary flavokawain A on prostate cancer tumor growth and progression in the TRAMP mice with FVB/N genetic background. Mice were fed either with control (AIN-76A) or AIN-76A diet supplemented with FKA [0.6% (w/w)] or KRE [0.6% (w/w)] for 3 weeks and water ad libitum. Body weights were recorded weekly and diet consumption was recorded twice a week throughout the study. At the end of the experiment, mice were sacrificed by carbon dioxide asphyxiation, and blood samples and organs were collected for further analyses. Use of mice and their care for this study was specifically approved by the University of California, Irvine Institutional Animal Care and Use Committee (IACUC; protocol number 2007-2741).
2.3. Histological analysis
For histological analysis tissue specimens were fixed for 24 h in buffered formaldehyde solution (3.7% in PBS) at room temperature, dehydrated by graded ethanol and embedded in paraffin. Tissue sections (thickness 5 m) were deparaffinized with xylene and stained with eosin/hematoxylin (H&E). Digital images were captured and analyzed with a Nikon Eclipse TE2000-S microscope (magnification, 100×).
2.4. Biochemical serum analysis [26]
Plasma samples were sent to IDEXX Laboratories (Irvine, CA) for preclinical services for analysis of plasma levels of alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), creatine phosphokinase (CPK), lactic acid dehydrogenase (LDH), lipase, albumin, creatinine, cholesterol, glucose, and lipemia index.
2.5. Cell proliferation and cytotoxicity assay
The cytotoxic effects of FKA on normal human small intestinal epithelial cells (FHS) and murine bone marrow cells were tested using the cell counting kit-8 (Kumamoto, Japan) following the kit instruction. FHS and murine bone marrow cells were exposed to 0.1% DMSO or different concentrations of FKA for 72 h. Premixed water-soluble tetrazolium salt (WST-1) was added into culture media and measured at 450 nm for densities of viable cells.
2.6. Colony formation assay
Murine bone marrow cells were isolated from 6 to 8 weeks old Balb/c mice according to previously reported methods [27]. After bone marrow cells were isolated, the yield and viability of cells were determined by Trypan blue exclusion method. A total number of 2 × 104 cells were seeded in a 6-well plate containing Colony Gel™ 1201 Mouse Base Medium (Reachbio), and then treated with FKA and Adriamycin at indicated concentrations for 2 weeks. The number of colonies was determined with an inverted phase-contrast microscope at 40× magnification. A group of >10 cells was counted as a colony.
2.7. Animal treatment with FKA for phase II enzyme studies
Seven-week-old mice were acclimatized for one week before use in the present study and fed a Purina chow diet and water ad libitum. FKA at doses of 100 and 200 mg/kg body weight/day dissolving in 0.5% carboxymethylcellulose sodium salt (CMC) was administered to mice by oral gavage. The control mice were orally given the same amount of 0.5% CMC solution. Each treatment group at each time point had five mice. These FKA treatments were given once in the morning daily and 24 h after 3, 7, 15 days mice in each group were sacrificed. The liver, lung, stomach, bladder, kidney and prostate were removed and immediately placed in ice-cold 0.1 M phosphate buffer, pH 7.4. Tissue were cleaned properly, minced and homogenized in the same buffer and 10,000 g supernatant fractions were prepared to determine the activities of phase II enzymes: Glutathione S-transferase (GST) and quinone reductase (QR). GST activity was determined according to the kit instruction using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. QR activity was determined as described by Benson et al. [28] using 2,6-dichlorophenol-indophenol (DCP-IP) as electron acceptor.
2.8. Statistics
Microsoft Excel software was used to compute mean and standard deviations of all quantitative data. Cell viability comparisons between treated and untreated (control) cells were accomplished using either analysis of variance (ANOVA) or Student's t-test. All statistical measures were two-sided, and P-values <0.05 were considered to be statistically significant.
3. Results
3.1. Dietary feeding of FKA did not affect food consumption and body weight of mice
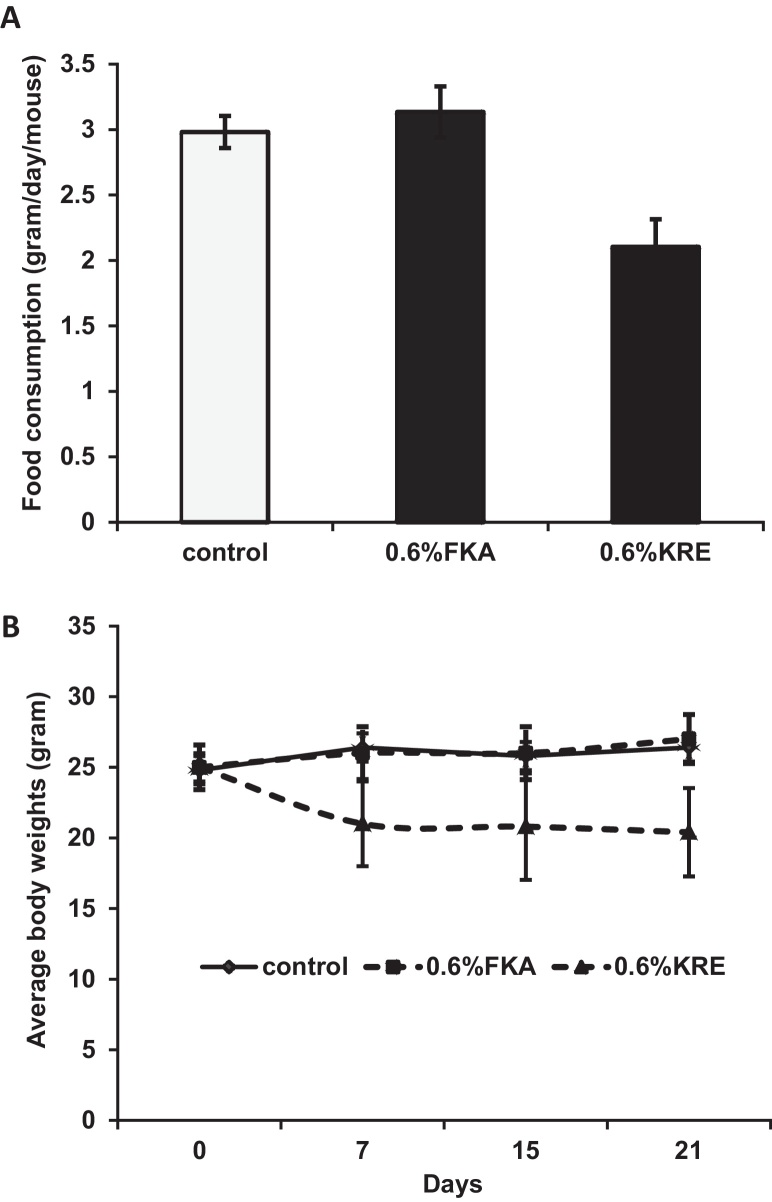
To study potential toxic effects of orally administrated FKA and KRE in vivo, mice were fed with AIN-76 A diet or AIN-76 A supplemented with 0.6% (w/w) FKA or KRE daily for 3 weeks. Fig. 1A shows that mice in the control group consumed food about 2.981 ± 0.123 g daily, while those in the KRE and FKA groups ate food about 2.104 ± 0.211 and 3.134 ± 0.195 g daily, respectively. KRE significantly reduced the daily food consumption compared to control diet and to diet supplemented with 0.6% FKA (Ps < 0.05). FKA appears to slightly increase the daily food consumption of mice. Final body weights were 26.4 ± 2.190 g for control mice, 20.4 ± 3.130 g for KRE-fed mice and 27.0 ± 1.732 for FKA-fed mice (Fig. 1B). KRE fed mice have a significantly less body weight gain over time compared to mice fed with control and FKA food (Ps < 0.05). However, there was no obvious difference between control and KRE or FKA fed mice in fecal excrements in terms of quantity, shape and consistency. There were also no obvious clinical symptoms (i.e. abnormal grooming behavior, abnormal shyness or lethargy, lameness, lethargy, anorexia) or deaths during the experimental period in all experimental mice.
Fig. 1.
The effect of dietary feeding of FKA or KRE on food consumption and body weight of mice. Mice (n = 6) were fed with AIN-76 A diet or AIN-76 A supplemented with 0.6% (w/w) FKA or KRE daily for 3 weeks. Mouse food consumption were recorded twice a week and body weight were recorded weekly, (A) mean daily food intake of mice ± standard deviation (SD) and (B) mean body weight of mice ± SD.
3.2. Effect of oral administration of FKA or KRE on function and homoeostasis of inner organs
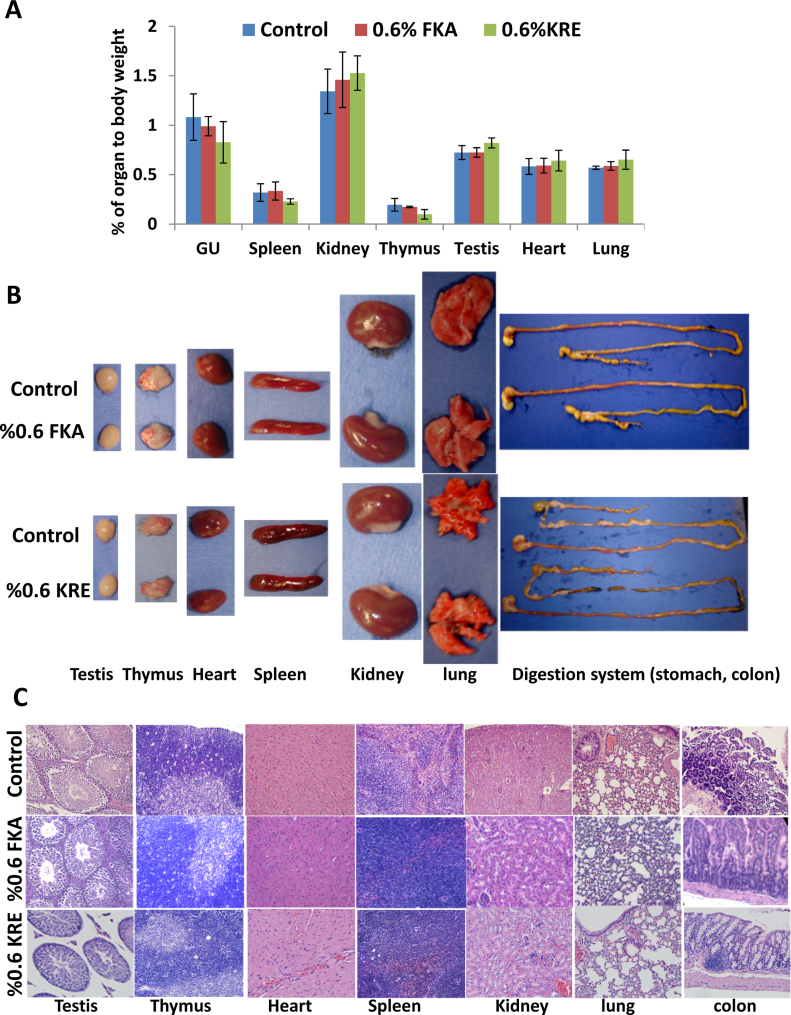
To determine whether FKA or KRE intake affects function and homeostasis of inner organs in mice fed with control or FKA diet, all major organs were inspected for frank toxicity and weighed. In addition, systematic necropsy for gross and microscopic examination was carried out and blood was collected for analyzing indicating markers of organ function. Fig. 2A shows that dietary feeding of FKA for three weeks did not change ratios of organ to body weight or organ weight [including heart, lung, spleen, kidney, thymus, testis and genitourinary (GU, prostate, seminal vesicles and bladder)], while KRE administration significantly increased the ratio of liver and testis to body weight and decreased ratios of thymus, spleen and GU to body weight (Ps < 0.05, ANNOVA test).
Fig. 2.
Macroscopical and microscopical examination of inner organs after FKA or KRE feeding. Mice were treated as described above. (A) Mean percentage of organ to body weight ± SD, (B) representative photographs of lung, heart, thymus, spleen, kidney (from left to right) and (C) H&E staining of different organ tissues, magnification 100×.
Gross examination demonstrated that FKA neither affected relative sizes of heart, lung, spleen, kidney, thymus, colon and testis or caused any visible abnormality (e.g. hydronephrosis, change of organ color) compared to control diet (Fig. 2B). Microscopical analysis revealed no inflammation, fibrosis, atrophy, alteration of parenchyma and mucosa or atypical epithelia in the tissue from these organs (Fig. 2C).
For serum markers of toxicity, lipase activity in the serum as a marker for the function of pancreas, creatine phosphokinase (CPK) as a marker for skeletal muscle function and potential acute poisoning, serum creatinine as a maker for kidney function, lactate dehydrogenase (LDH) as a marker for general toxicity and cholesterol were assessed, Table 1 shows that there is no significant difference between control and KRE or FKA dietary groups in the average levels of these markers for organ functions.
Table 1.
Serum analysis of control, KRE and FKA treated mice.
| Parameter | Control | KRE | FKA | P-value |
|---|---|---|---|---|
| Creatinine (mg/dL) | 0.20 ± 0.01 | 0.18 ± 0.08 | 0.18 ± 0.04 | 0.65#; 0.41$ |
| Lipase (U/L) | 71.75 ± 5.9 | 155.6 ± 183.7 | 83.2 ± 14.9 | 0.40#; 0.19$ |
| CHOLESTEROL (mg/dL) | 119.2 ± 52.6 | 156.2 ± 50.0 | 117.6 ± 37.9 | 0.24#; 0.96$ |
| Creatine phosphokinase (IU/L) | 289.4 ± 115.8 | 398.8 ± 152.2 | 248.6 ± 52.7 | 0.24#; 0.49$ |
| LDH (IU/L) | 345.25 ± 115.4 | 728.2 ± 455.2 | 899 ± 773.1 | 0.15#; 0.20$ |
| Alkaline phosphatase (units/mg) | 132.8 ± 64.3 | 128.6 ± 68.1 | 127.8 ± 39.4 | 0.91#; 0.90$ |
The data shown are means ± SD of six independent mice.
P values for KRE versus control.
$P values for KRE versus control.
3.3. Effect of dietary consumption of FKA or KRE on liver function and homoeostasis
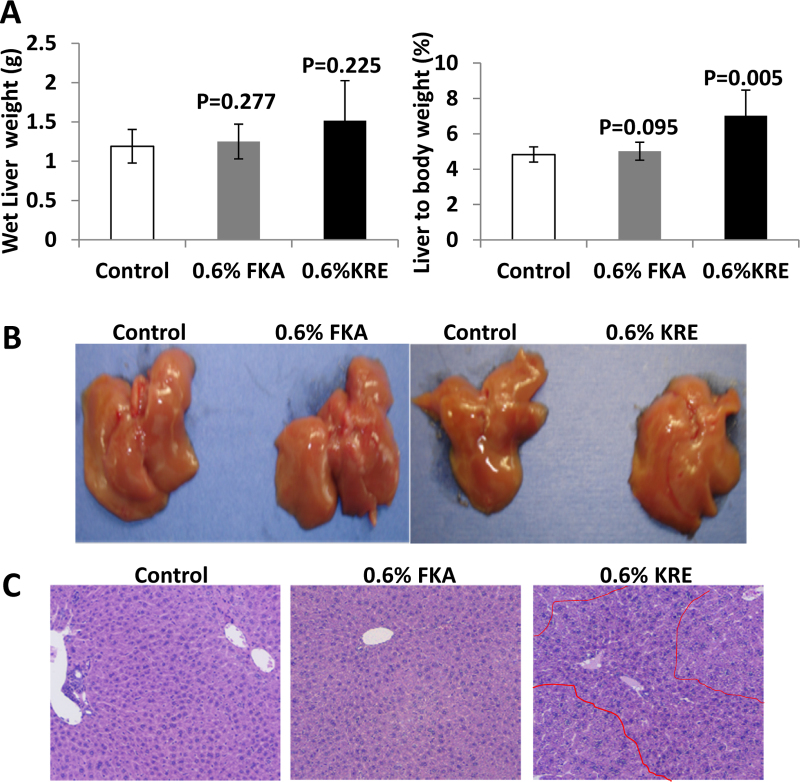
Although experimental and epidemiological data have demonstrated the potential of kava and its active components as cancer chemopreventive agents, hepatotoxicity remains to be a major concern for the use of kava or kava components for cancer prevention. We therefore evaluated the effect of dietary feeding of FKA or KRE on the liver. Fig. 3A shows that neither absolute wet liver weight nor liver to body weight ratio differed between control and FKA groups, whereas that dietary feeding of KRE increased both wet liver weight and liver to body weight ratio. Macroscopical examination of the livers revealed no signs of hepatotoxicity among all experimental groups (Fig. 3B). Pathological analysis demonstrates a regular structure of normal liver parenchyma with small portal tracts, regular reticulin network and low variation in hepatocellular nuclei size in both control and FKA treatment groups. There is no identifiable inflammation or steatosis (Fig. 3C). However, dietary feeding of 0.6% KRE resulted in a significant appearance of proliferating nodules consisting of closely packed hepatocytes in the liver (Fig. 3D).
Fig. 3.
Analysis of potential hepatotoxic effects of FKA and KRE fed mice. Mice were fed as described above. (A) Absolute liver weight and (B) liver-to-body-weight-ratio of FKA and KRE treated and control mice after 3 weeks (mean ± SD). (C) Representative photographs of the whole liver and (D) histological analysis of sections of hepatic tissue (H&E staining; bars represent 500 μm in the upper and 100 μm in the lower row).
Biochemical analyses of serum parameters indicative of liver damage and hepatic synthesis capacity shows that there are no significant difference in the average serum levels of ALT, AST and alkaline phosphatase (ALP), as well as albumin, cholesterol and glucose among mice of KRE, FKA and control treatment groups (Table 2).
Table 2.
Liver relevant serum parameters of control, KRE and FKA treated mice.
| Parameter | Control | KRE | FKA | P-value |
|---|---|---|---|---|
| ALT (U/L) | 106.4 ± 148.7 | 261.8 ± 346.4 | 164.6 ± 159.0 | 0.38#; 0.57$ |
| AST (U/L) | 226.0 ± 265.9 | 314.4 ± 296.9 | 262.8 ± 225.6 | 0.63#; 0.82$ |
| Albumin (g/L) | 3.02 ± 0.16 | 2.9 ± 0.41 | 3.1 ± 0.21 | 0.35#; 0.70$ |
| Glucose (mg/dL) | 269.2 ± 97.7 | 190.4 ± 44.9 | 243.4 ± 45.7 | 0.14#; 0.60$ |
The data shown are means ± SD of six independent mice.
P values for KRE versus control.
$P values for KRE versus control.
3.4. The toxic effect of FKA or KRE on human normal small intestinal epithelial cells and mouse bone marrow cells
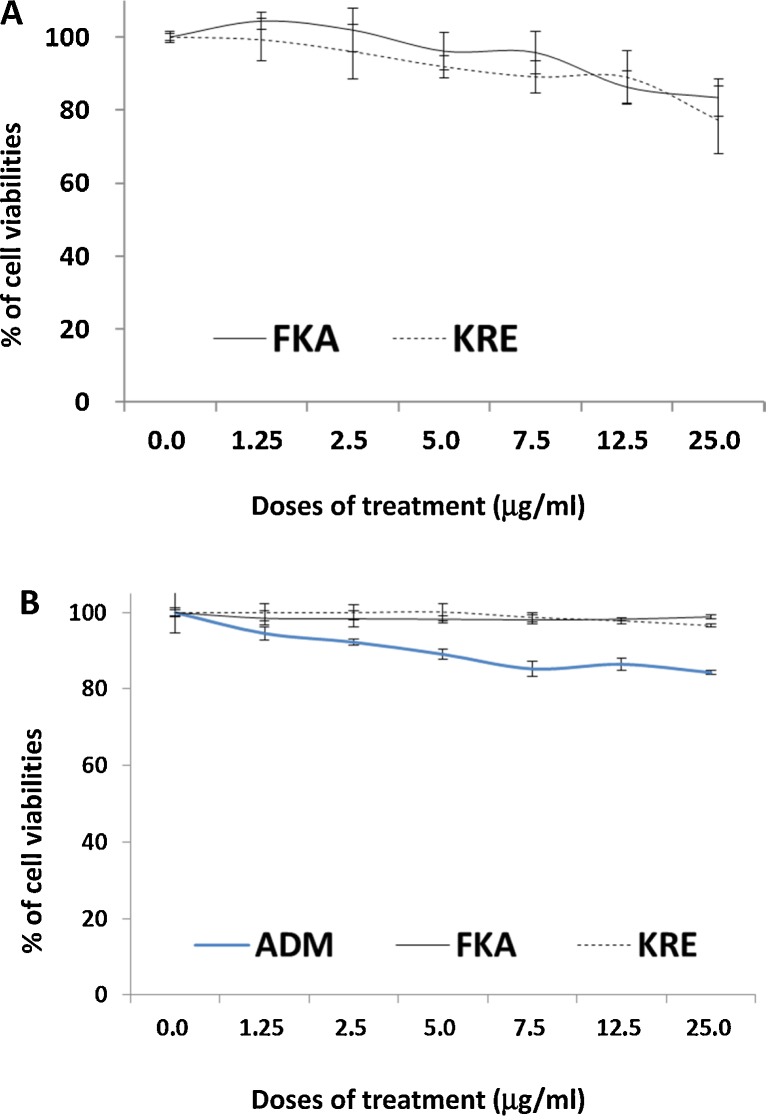
Fig. 4A shows that both FKA and KRE at a dose of up to 25 μg/ml have very low or minimal toxicity to fast-growing human normal small intestinal epithelial cells. This dose of FKA or KRE has been shown to completely inhibit the growth of cancer cell lines.
Fig. 4.
Analysis of potential toxic effect of FKA, KRE and Adriamycin (ADM) on normal small intestinal epithelial cell (FHS) and mouse bone marrow cells. FHS and mouse bone marrow Cells were treated with 0.1% DMSO, FKA, KRE, or ADM at indicated doses for 72 h. After these treatments, cell densities were measured by MTT assay. Each point is the mean percentage of cell density values relative to vehicle control; bars, SD. (A) FKA and KRE have minimal effect on the growth of FHS cells up to 25 μg/ml. (B) Compared with ADM, FKA and KRE showed significantly less bone marrow inhibition effect.
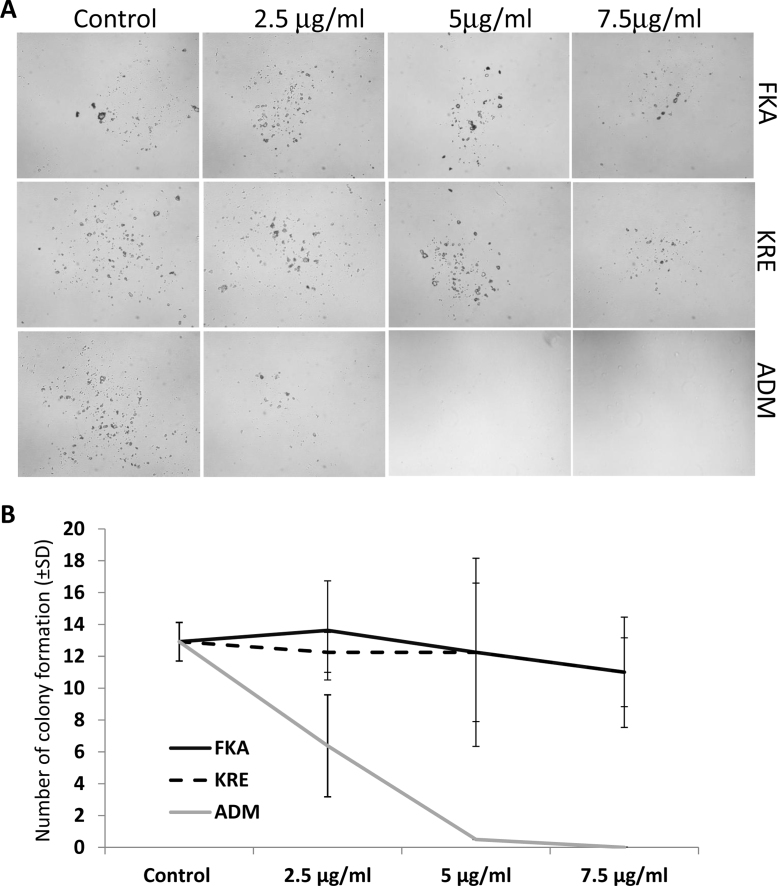
In addition, the potential toxicity of FKA or KRE to bone marrow was investigated by using mouse bone marrow cells. No significant growth inhibitory effects on bone marrow cells were observed following FKA and KRE treatments for 72 h (Fig. 4B). Bone marrow cell colony formation revealed that there was also no significant difference in the number of colonies after FKA and KRE treatments (Fig. 5A and B, Ps > 0.05). However, a significant inhibition of colony formation was noted with Adriamycin treatment at all concentrations (Fig. 5A and B, Ps < 0.05–0.01).
Fig. 5.
Analysis of potential toxic effect of FKA, KRE and Adriamycin (ADM) on colony formation of mouse bone marrow cells. (A) Representative microphotography, taken at 14 days after treatment of mouse bone marrow stem cell colonies. (B) The mean number of colony formation after treatment of mouse bone marrow stem cells for 14 days; bars, SD.
3.5. Enhancement of phase II enzyme activities by FKA
The enhancement of phase II enzyme activities has been thought to be an effective mechanism for prevention of cancer by chemopreventive agents [28]. FKA contains an electrophilic α,β-unsaturated ketone, which might react with intracellular GSH to form conjugates and then transcriptionally induce phase II enzyme activities for protection against carcinogenesis [29]. To investigate this possibility, studies were also performed to assess the effect of orally administered FKA on both GST and QR enzyme activities. As shown in Table 3, Table 4, oral administration of FKA resulted in a different degree of increase in both GST and QR activities in all the tissues examined. In the case of QR activity, compared with vehicle-treated controls, treatment with 100 and 200 mg/kg doses of FKA for 3, 7 and 15 days resulted in 11.1–248% and 12.5–258% increases (Ps < 0.05, Student's t-test) in enzyme activity. The observed increase in QR activity was more pronounced in liver, prostate and bladder and accounted for the 110, 140% and 258% increases (P < 0.001, Student's t-test) over control for 200 mg/kg dose, respectively (Table 3). For GST activity, compared with vehicle-treated controls, the increases in GST activity were also significant in liver and bladder at the 100 mg/kg or 200 mg/kg dose of FKA after 3 days treatment (Table 4, Student's t-test, P < 0.01). In general, the increases in GST activity in liver, bladder and prostate were not as profound as those in QR activity (Table 3, Table 4). There is no obvious dose- and time-dependent effect of FKA in induction of phase II enzyme activities. However, oral administration of FKA resulted in less pronounced increases in GST and QR activities in lung tissues than those in liver, prostate and bladder tissues. The maximum increase in QR activity by FKA in lung tissue is 125% versus 248% in liver and 258% in bladder (ANOVA test, P < 0.05). The maximum increase in GST activity by FKA in lung tissue is 13% versus 61% in liver tissues and 71% in bladder tissues (ANOVA test, P < 0.05).
Table 3.
Oral administration of FKA to mice induces QR activities in different tissues.
| FKA (mg/kg) | QR (nmol DCP-IP reduced/min/mg protein) |
||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 15 (days of administration) | ||
| Liver | 0 (control) | 2648.06 ± 183.35 | 2436.80 ± 1398.10 | 2741.66 ± 137.60 | 2765.72 ± 2093.37 |
| 100 | 4101.02 ± 1105.19 | 3527.77 ± 517.85* | 9634.45 ± 5313.81* | ||
| 200 | 8504.29 ± 3470.56* | 4068.79 ± 232.22* | 7068.51 ± 3294.60* | ||
| Bladder | 0 | 3449.71 ± 209.77 | 3273.61 ± 1415.96* | 3393.723 ± 443.62 | 3681.79 ± 100.181 |
| 100 | 11737.22 ± 4084.48* | 3467.52 ± 688.35 | 5170.27 ± 739.32* | ||
| 200 | 10511.25 ± 1472.25* | 3770.84 ± 1072.36 | 3089.84 ± 768.84 | ||
| Prostate | 0 | 2802.91 ± 64.67 | 2756.19 ± 1587.58 | 2876.72 ± 2198.77 | 2775.82 ± 1878.86 |
| 100 | 2310.67 ± 226.59 | 5523.26 ± 969.70* | 6807.09 ± 1674.26* | ||
| 200 | 2337.18 ± 1150.77 | 5119.12 ± 4551.13 | 6411.29 ± 1356.39* | ||
| Lung | 0 | 4870.35 ± 39.78 | 4851.78 ± 527.02 | 4916.02 ± 2880.84 | 4843.25 ± 1181.73 |
| 100 | 3857.17 ± 1549.93 | 4524.02 ± 5647.48 | 3473.61 ± 1628.91 | ||
| 200 | 4339.79 ± 88.12 | 11079.75 ± 1966.66* | 8950.10 ± 2933.21 | ||
| Stomach | 0 | 60479.048 ± 3081.32 | 64021.47 ± 5996.13 | 59000.77 ± 14874.92 | 58416.41 ± 12850.78 |
| 100 | 57294.48 ± 11656.99 | 50044.99 ± 15353.51 | 91235.17 ± 20875.47* | ||
| 200 | 74057.26 ± 30492.55 | 59889.06 ± 9694.78 | 69008.18 ± 11494.82 | ||
| Small intestine | 0 | 40637.76 ± 1523.47 | 41885.29 ± 4398.57 | 38927.15 ± 2909.39 | 41100.84 ± 18778.52 |
| 100 | 74065.44 ± 8922.01 | 43262.27 ± 1309.49* | 54448.36 ± 9452.83* | ||
| 200 | 60568.51 ± 2085.84 | 43810.33 ± 472.22* | 47506.90 ± 10479.29 | ||
The data shown are means ± SD of five independent mice.
Statistically significant versus control group, P < 0.05 (t-test).
Table 4.
Oral administration of FKA to mice induces GST activities in different tissues.
| FKA (mg/kg) | GST (nmol/min/mg) |
||||
|---|---|---|---|---|---|
| 0 | 3 | 7 | 15(days of administration) | ||
| Liver | 0 | 729.47 ± 33.87 | 740.10 ± 112.58 | 691.56 ± 86.74 | 756.76 ± 83.00 |
| 100 | 1191.81 ± 235.47* | 711.04 ± 25.47 | 1203.04 ± 243.90* | ||
| 200 | 1015.37 ± 108.39* | 700.25 ± 62.66 | 1013.56 ± 107.02* | ||
| Bladder | 0 | 450.71 ± 18.20 | 444.29 ± 44.89 | 471.15 ± 108.47 | 436.58 ± 55.70 |
| 100 | 746.65 ± 134.09* | 520.07 ± 99.02 | 488.46 ± 63.37 | ||
| 200 | 759.97 ± 67.51* | 564.10 ± 76.31 | 434.44 ± 48.89 | ||
| Prostate | 0 | 208.93 ± 4.62 | 205.41 ± 85.90 | 207.2352 ± 11.59 | 214.17 ± 23.70 |
| 100 | 233.77 ± 86.73 | 198.486 ± 15.16 | 230.93 ± 41.64 | ||
| 200 | 239.19 ± 90.09 | 201.506 ± 23.59 | 220.78 ± 19.94 | ||
| Lung | 0 | 229.89 ± 10.54 | 217.91 ± 11.30 | 237.75 ± 44.33 | 234.02 ± 101.90 |
| 100 | 188.04 ± 43.10 | 228.28 ± 35.06 | 230.62 ± 20.06 | ||
| 200 | 233.59 ± 28.391 | 275.74 ± 10.79 | 265.59 ± 56.67* | ||
| Stomach | 0 | 169.84 ± 6.27 | 163.94 ± 27.03 | 176.44 ± 64.28 | 169.14 ± 13.58 |
| 100 | 194.46 ± 13.51 | 211.20 ± 31.71 | 223.55 ± 15.44* | ||
| 200 | 232.99 ± 46.97* | 214.10 ± 14.58 | 220.38 ± 18.62* | ||
| Small intestine | 0 | 242.88 ± 16.18 | 224.68 ± 39.81 | 248.30 ± 34.72 | 255.67 ± 42.49 |
| 100 | 273.20 ± 19.50* | 393.39 ± 105.97* | 307.94 ± 17.81* | ||
| 200 | 296.34 ± 22.11* | 394.84 ± 50.59* | 274.42 ± 39.89 | ||
The data shown are means ± SD of five independent mice.
Statistically significant versus control group, P < 0.05 (t-test).
4. Discussion
Traditional kava preparation has been considered to be safe for daily use in the South Pacific Island Nations for thousands of years until there had been several reports of suspected commercial kava extracts-induced acute liver toxicity in Western countries, including Europe and the USA between 1990 and 2002 [30]. Therefore, the potential hepatotoxicity of KRE has recently been examined by several groups in rats or mice, as well as in human [31], [32], [33], [34], [35], [36], [37], [38], [39]. However, the results from these studies are conflicting and kava hepatotoxicity remains a matter of debate. Most of these studies [34], [35], [37], [38], [39] have shown that KRE failed to result in toxicity and increases in serum markers (i.e. ALT and AST) of hepatotoxicity in rat and mice. In addition, clinical studies showed that compared with abstainers and non-drinkers in Australian aboriginal and Tongan populations, recent kava drinkers had significantly elevated serum levels of the cholestatic enzymes γ-glutamyl transferase (GGT) and alkaline phosphatase (ALP) without increased ALT or AST levels [31], [32], [33]. These results suggested biliary inflammation rather than hepatocellular damage in kava drinkers. In a National Toxicology Program study, rats and mice that were fed with 0.125–2 g commercial kava root extracts/kg body weight by gavage for two weeks, thirteen weeks or two years exhibited dose- and time-related increases in liver weights and incidences of hepatocellular hypertrophy [36]. The manifestation of this hepatotoxicity is also not consistent with the reported hepatotoxicity in human, which is of idiosyncratic, unpredictable, and dose-independent nature and was thought to be due to aberrant drug metabolism in a small portion of kava users in the Western countries [18], [37], [40]. Histologically, the kava hepatotoxicity in human is more related to hepatocellular liver disease, including necrosis, hepatitis, cirrhosis, and liver failure, while the kava hepatotoxicity in rodents was characterized by increased incidences of hepatocellular hypertrophy, which is often associated with an adaptive response to enzyme induction by xenobiotics [36], [40], [41]. Our study showed that commercial KRE increased liver weight and resulted in a significant appearance of proliferating nodules, further suggesting that the commercial KRE act through enzyme induction to induce liver lesions in mice. However, our results still cannot explain the potential toxicity of KRE in human.
The complex kava extracts contain many bioactive ingredients. Currently, there is no sufficient evidence for a causative role of any individual kava component, such as pipermethystine and flavokawain B, in kava hepatotoxicity. FKA is the principal chalcone in the Kava plant [21]. In general, chalcones are well tolerant in many animal experiments and considered non-toxic. Citrus fruits and apples are rich dietary sources of chalcones [42]. The maximum tolerant doses of some chalcones in rodents were shown to be more than 1–3 g/kg body weight [43]. Previous studies from us and other groups have demonstrated that FKA at concentrations significantly inhibits the growth of many types of cancer cell lines with minimal effect on the growth of normal cells derived from different types of tissues, including breast, liver, prostate, fibroblast, intestine, and bone marrows (Fig. 4 and data not shown). We have recently shown that 0.6% FKA feeding of UPII-SV40T transgenic mice for 290 days did not result in any noticeable toxicity. The maximum detected FKA concentration in plasma of these mice is about 1.8 μM. However, Li et al. [22] reported that the IC50s of FKA to human normal liver cells L-02 and human hepatoma cells HepG2 cells are more than 100 μM. Therefore, it is unlikely that the in vitro concentrations of FKA for hepatocellular toxicity can be applied to the in vivo situations. In this study, mice that were fed with standard diet supplemented with 0.6% FKA to achieve a daily dose of about 960 mg FKA/kg body for 3 weeks exhibited neither absolute wet liver weight increase nor liver to body weight ratio gain, as well as no changes in food and water consumption. Pathological analysis demonstrates a regular structure of normal liver parenchyma with small portal tracts; regular reticulin network and low variation in hepatocellular nuclei size without any identifiable inflammation or steatosis. The examination of liver function and homeostasis serum biomarkers (i.e. ALT, AST, albumin, glucose and others) in FKA fed mice also revealed no liver damage and no changes in hepatic synthesis capacity. Taken together, these results indicated a satisfactory/excellent safety profile of FKA.
Phase II enzymes, including GST and QR, play a critical role in detoxification of activated carcinogenic intermediates [44]. Consequently, induction of phase II enzyme activities by chemopreventive agents has become a major mechanism for alteration of carcinogen metabolism and cancer prevention. In this study, increases in phase II enzyme activity induced by FKA were clearly evident in the mouse liver, prostate and bladder. This result may form the basis for more detailed studies in the future to evaluate the cancer preventive and interventive effects of FKA, the predominant chalcone in the kava plant, in experimental models of carcinogenesis representing liver, prostate and bladder sites.
In conclusion, oral administration of FKA to mice in a dose as high as 960 mg/kg body per day did not cause any toxicity or adverse effects. In particular, the potential hepatotoxicity of FKA could be ruled out. FKA significantly induces activity of phase II enzymes, including GST and QR, in the mouse liver, prostate and bladder. FKA preferably inhibits the growth of cancer cells with no or minimal effects on the growth of several types of normal cells. Therefore, FKA is an excellent candidate for cancer chemoprevention studies. Further studies are needed to assess the cancer preventive and anticarcinogenic effects of flavokawain A in different cancer models for prostate and bladder cancer prevention.
Conflict of interest
The authors declare no conflict of interests.
Acknowledgements
This work was in part supported by NIH award 5R01CA122558-05 and 1R21CA152804-01A1 (to X.Z.).
Footnotes
Available online 28 February 2014
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int. J. Cancer. 2008;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Steiner G.G. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000;59:420–422. [PubMed] [Google Scholar]
- 3.Johnson T.E., Kassie F., O'Sullivan M.G., Negia M., Hanson T.E., Upadhyaya P., Ruvolo P.P., Hecht S.S., Xing C. Chemopreventive effect of kava on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Cancer Prev. Res. (Phila) 2008;1:430–438. doi: 10.1158/1940-6207.CAPR-08-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson T.E., Hermanson D., Wang L., Kassie F., Upadhyaya P., O'Sullivan M.G., Hecht S.S., Lu J., Xing C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am. J. Chin. Med. 2011;39:727–742. doi: 10.1142/S0192415X11009202. [DOI] [PubMed] [Google Scholar]
- 5.Li X., Liu Z., Xu X., Blair C.A., Sun Z., Xie J., Lilly M.B., Zi X. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PLOS ONE. 2012;7:e31213. doi: 10.1371/journal.pone.0031213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaik A.A., Hermanson D.L., Xing C. Identification of methysticin as a potent and non-toxic NF-kappaB inhibitor from kava, potentially responsible for kava's chemopreventive activity. Bioorg. Med. Chem. Lett. 2009;19:5732–5736. doi: 10.1016/j.bmcl.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Y., Simoneau A.R., Xie J., Shahandeh B., Zi X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev. Res. (Phila) 2008;1:439–451. doi: 10.1158/1940-6207.CAPR-08-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Li X., Liu Z., Simoneau A.R., Xie J., Zi X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer. 2010;127:1758–1768. doi: 10.1002/ijc.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zi X., Simoneau A.R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–3486. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 10.Singh Y.N. Kava: an overview. J. Ethnopharmacol. 1992;37:13–45. doi: 10.1016/0378-8741(92)90003-a. [DOI] [PubMed] [Google Scholar]
- 11.Kapadia G.J., Azuine M.A., Tokuda H., Hang E., Mukainaka T., Nishino H., Sridhar R. Inhibitory effect of herbal remedies on 12-O-tetradecanoylphorbol-13-acetate-promoted Epstein-Barr virus early antigen activation. Pharmacol. Res. 2002;45:213–220. doi: 10.1006/phrs.2001.0936. [DOI] [PubMed] [Google Scholar]
- 12.Triolet J., Shaik A.A., Gallaher D.D., O'Sullivan M.G., Xing C. Reduction in colon cancer risk by consumption of kava or kava fractions in carcinogen-treated rats. Nutr. Cancer. 2012;64:838–846. doi: 10.1080/01635581.2012.689917. [DOI] [PubMed] [Google Scholar]
- 13.Clouatre D.L. Kava kava: examining new reports of toxicity. Toxicol. Lett. 2004;150:85–96. doi: 10.1016/j.toxlet.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Eskander R.N., Randall L.M., Sakai T., Guo Y., Hoang B., Zi X. Flavokawain B, a novel, naturally occurring chalcone, exhibits robust apoptotic effects and induces G2/M arrest of a uterine leiomyosarcoma cell line. J. Obstet. Gynaecol. Res. 2012;38:1086–1094. doi: 10.1111/j.1447-0756.2011.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai T., Eskander R.N., Guo Y., Kim K.J., Mefford J., Hopkins J., Bhatia N.N., Zi X., Hoang B.H. Flavokawain B, a kava chalcone, induces apoptosis in synovial sarcoma cell lines. J. Orthop. Res. 2012;30:1045–1050. doi: 10.1002/jor.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warmka J.K., Solberg E.L., Zeliadt N.A., Srinivasan B., Charlson A.T., Xing C., Wattenberg E.V. Inhibition of mitogen activated protein kinases increases the sensitivity of A549 lung cancer cells to the cytotoxicity induced by a kava chalcone analog. Biochem. Biophys. Res. Commun. 2012;424:488–492. doi: 10.1016/j.bbrc.2012.06.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechtenberg M., Schmidt Q.B.M., Nahrstedt A. Is the alkaloid pipermethystine connected with the claimed liver toxicity of Kava products? Pharmazie. 2008;63:71–74. [PubMed] [Google Scholar]
- 18.Teschke R., Sarris J., Lebot V. Contaminant hepatotoxins as culprits for kava hepatotoxicity – fact or fiction? Phytother. Res. 2012;27:472–474. doi: 10.1002/ptr.4729. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Rowe A., Braet F., Ramzan I. Macrophage depletion ameliorates kavalactone damage in the isolated perfused rat liver. J. Toxicol. Sci. 2012;37:447–453. doi: 10.2131/jts.37.447. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P., Gross S., Liu J.H., Yu B.Y., Feng L.L., Nolta J., Sharma V., Piwnica-Worms D., Qiu S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-kappaB and MAPK signaling pathways. FASEB J. 2010;24:4722–4732. doi: 10.1096/fj.10-163311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dharmaratne H.R., Nanayakkara N.P., Khan I.A. Kavalactones from Piper methysticum, and their 13C NMR spectroscopic analyses. Phytochemistry. 2002;59:429–433. doi: 10.1016/s0031-9422(01)00443-5. [DOI] [PubMed] [Google Scholar]
- 22.Li N., Liu J.H., Zhang J., Yu B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008;56:3876–3883. doi: 10.1021/jf073520n. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Xu X., Li X. KAVA chalcone, flavokawain A, inhibits urothelial tumorigenesis in the UPII-SV40T transgenic mouse model. Cancer Prev. Res. (Phila) 2013;6:1365–1375. doi: 10.1158/1940-6207.CAPR-13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cote C.S., Kor C., Cohen J., Auclair K. Composition and biological activity of traditional and commercial kava extracts. Biochem. Biophys. Res. Commun. 2004;322:147–152. doi: 10.1016/j.bbrc.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 25.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–666. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 26.Hillier S.M., Marquis J.C., Zayas B., Wishnok J.S., Liberman R.G., Skipper P.L., Tannenbaum S.R., Essigmann J.M., Croy R.G. DNA adducts formed by a novel antitumor agent 11beta-dichloro in vitro and in vivo. Mol. Cancer Ther. 2006;5:977–984. doi: 10.1158/1535-7163.MCT-05-0464. [DOI] [PubMed] [Google Scholar]
- 27.Soleimani M., Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat. Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- 28.Benson A.M., Hunkeler M.J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. U. S. A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park E.Y., Cho I.J., Kim S.G. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-gamma and retinoid X receptor heterodimer. Cancer Res. 2004;64:3701–3713. doi: 10.1158/0008-5472.CAN-03-3924. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention . 2002. Hepatic Toxicity Possibly Associated with Kava-containing Products—United States, Germany, and Switzerland; pp. 1999–2002. [PubMed] [Google Scholar]
- 31.Brown A.C., Onopa J., Holck P., Kaufusi P., Kabasawa D., Craig W.J., Dragull K., Levine A.M., Baker J.D. Traditional kava beverage consumption and liver function tests in a predominantly Tongan population in Hawaii. Clin. Toxicol. (Phila) 2007;45:549–556. doi: 10.1080/15563650701365875. [DOI] [PubMed] [Google Scholar]
- 32.Clough A.R., Bailie R.S., Currie B. Liver function test abnormalities in users of aqueous kava extracts. J. Toxicol. Clin. Toxicol. 2003;41:821–829. doi: 10.1081/clt-120025347. [DOI] [PubMed] [Google Scholar]
- 33.Clough A.R., Jacups S.P., Wang Z., Burns C.B., Bailie R.S., Cairney S.J., Collie A., Guyula T., McDonald S.P., Currie B.J. Health effects of kava use in an eastern Arnhem Land Aboriginal community. Intern. Med. J. 2003;33:336–340. doi: 10.1046/j.1444-0903.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 34.DiSilvestro R.A., Zhang W., DiSilvestro D.J. Kava feeding in rats does not cause liver injury nor enhance galactosamine-induced hepatitis. Food Chem. Toxicol. 2007;45:1293–1300. doi: 10.1016/j.fct.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Lim S.T., Dragull K., Tang C.S., Bittenbender H.C., Efird J.T., Nerurkar P.V. Effects of kava alkaloid, pipermethystine, and kavalactones on oxidative stress and cytochrome P450 in F-344 rats. Toxicol. Sci. 2007;97:214–221. doi: 10.1093/toxsci/kfm035. [DOI] [PubMed] [Google Scholar]
- 36.National Toxicology Program Toxicology and carcinogenesis studies of kava kava extract (CAS No. 9000-38-8) in F344/N rats and B6C3F1 mice (Gavage Studies) Natl. Toxicol. Program Tech. Rep. Ser. 2012:1–186. [PubMed] [Google Scholar]
- 37.Russmann S., Lauterburg B.H., Barguil Y., Choblet E., Cabalion P., Rentsch K., Wenk M. Traditional aqueous kava extracts inhibit cytochrome P450 1A2 in humans: protective effect against environmental carcinogens? Clin. Pharmacol. Ther. 2005;77:453–454. doi: 10.1016/j.clpt.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Singh Y.N., Devkota A.K. Aqueous kava extracts do not affect liver function tests in rats. Planta Med. 2003;69:496–499. doi: 10.1055/s-2003-40658. [DOI] [PubMed] [Google Scholar]
- 39.Sorrentino L., Capasso A., Schmidt M. Safety of ethanolic kava extract: results of a study of chronic toxicity in rats. Phytomedicine. 2006;13:542–549. doi: 10.1016/j.phymed.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Teschke R. Kava hepatotoxicity—a clinical review. Ann. Hepatol. 2010;9:251–265. [PubMed] [Google Scholar]
- 41.Maronpot R.R., Yoshizawa K., Nyska A., Harada T., Flake G., Mueller G., Singh B., Ward J.M. Hepatic enzyme induction: histopathology. Toxicol. Pathol. 2010;38:776–795. doi: 10.1177/0192623310373778. [DOI] [PubMed] [Google Scholar]
- 42.Nelson J.A., Falk R.E. The efficacy of phloridzin and phloretin on tumor cell growth. Anticancer Res. 1993;13:2287–2292. [PubMed] [Google Scholar]
- 43.Baba M., Asano R., Takigami I., Takahashi T., Ohmura M., Okada Y., Sugimoto H., Arika T., Nishino H., Okuyama T. Studies on cancer chemoprevention by traditional folk medicines XXV, inhibitory effect of isoliquiritigenin on azoxymethane-induced murine colon aberrant crypt focus formation and carcinogenesis. Biol. Pharm. Bull. 2002;25:247–250. doi: 10.1248/bpb.25.247. [DOI] [PubMed] [Google Scholar]
- 44.Hayes J.D., Pulford D.J. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]