Supplemental Digital Content is available in the text.
Keywords: bipolar disorder, borderline personality disorder, CACNA1C, comorbidity, genetic overlap
Abstract
The objective of this study was to investigate the hypothesis that borderline personality disorder (BPD) and bipolar disorder (BD) share genetic variation through analysis of known genetic risk factors for BD in a well-characterized BPD case–control cohort. Genotyping of five genome-wide significant variants identified for BD (in CACNA1C, ANK3, and ODZ4) was performed in 673 BPD cases and 748 controls. A nominally significant association with BPD was found for rs1006737 in CACNA1C (P=0.0498). Sex-specific analysis showed that this signal was present only in women. This is the first report of an association between a BD risk gene and BPD where selection was not based on a priori hypotheses about its function, but on an unbiased hypothesis-free screening of the genome. Genome-wide association data of large samples of BPD are warranted and will eventually identify new risk genes and the overlap between BPD and BD if it exists.
Introduction
Borderline personality disorder (BPD) is characterized by affective instability, emotion dysregulation, and poor interpersonal functioning (Lieb et al., 2004). BPD has a prevalence of 1–2%, and is associated with major psychosocial dysfunction and economic burden (Lieb et al., 2004).
At the time of writing, the etiology of BPD remains unclear (Lieb et al., 2004). Heritability estimates from family and twin studies range between 35 and 65% (Torgersen et al., 2000; Distel et al., 2009). To date, genetic research into BPD has been limited. Previous genetic studies of BPD involved small samples, and focused on candidate genes, for example from the serotonergic system (SLC6A4, TPH2, MAOA); the dopaminergic system (COMT, MAOA); and the neurotrophins (BDNF) (Calati et al., 2013). The results of these studies have been inconsistent. In contrast to other major psychiatric disorders, no genome-wide significant studies for BPD have been reported as yet.
An interesting feature of BPD in terms of the hunt for genetic factors is the high level of psychiatric comorbidity. In particular, individuals with BPD show high comorbidity, and a considerable overlap in terms of phenomenology, with bipolar disorder (BD) (Zimmerman and Morgan, 2013). Both BPD and BD are characterized by a tendency toward impulsivity, affective instability, recurrent suicidality, intense anger, and unstable interpersonal relationships. Thus, previous authors have challenged the BPD/BD dichotomy, and hypothesized that these two disorders may in fact have a common etiology (Akiskal, 2004). The nosological relationship between these two major psychiatric disorders is the subject of intense ongoing debate within the field (Coulston et al., 2012).
The phenomenological overlap and the high comorbidity between BD and BPD suggest the hypothesis of a common genetic background. This in turn suggests that investigation of genetic risk variants for BD in BPD samples is warranted. So far, no twin or family studies have provided conclusive results on whether there is a genetic overlap between the two disorders (Loranger et al., 1982; Pope et al., 1983).
The aim of the present study was to investigate genome-wide significant variants for BD located in the genes CACNA1C, ANK3, and ODZ4 (Ferreira et al., 2008; Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011) in well-characterized BPD case–control samples to determine the existence of a common genetic background.
Materials and methods
Participants
The present analyses involved 673 BPD patients and 748 controls (for details of recruitment sites and clinical assessment, see SDI, Supplemental digital content 1, http://links.lww.com/PG/A115). Written informed consent was obtained from all participants before inclusion. All study procedures conformed to the Code of Ethics of the World Medical Association (Declaration of Helsinki, 6th revision, 2008). The study was approved by the ethics committees of each study center. Demographic data for the patients and the controls are shown in Table S1a/b (SDI, Supplemental digital content 1, http://links.lww.com/PG/A115).
SNP selection
The genetic variants of interest were five genome-wide significant risk variants identified for BD: rs1006737 [P=7.0×10–8, (Ferreira et al., 2008)] [P=3.1×10–8, (Liu et al., 2011)] and rs4765913 [P=1.52×10–8, (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011)] in CACNA1C; rs10994336 [P=9.1×10–9, (Ferreira et al., 2008)] and rs10994397 [P=7.08×10–9, (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011)] in ANK3; and rs12576775 [P=4.40×10–8, (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011)] in ODZ4.
Genotyping
Genomic DNA was prepared from whole blood according to standard procedures. Selected single-nucleotide polymorphisms (SNPs) were genotyped using the iPLEX assay on the MassARRAY MALDI-TOF mass spectrometer (SEQUENOM, San Diego, California, USA) within the context of a larger study. For details, see SDI (Supplemental digital content 1, http://links.lww.com/PG/A115).
Statistical analysis
Data preparation and analysis were carried out using the following three software packages: PLINK, version 1.07 (http://pngu.mgh.harvard.edu/~purcell/plink/); PASW Statistics for Windows, version 18.0. (SPSS Inc., Chicago, Illinois, USA); and R, version 2.15.3 (http://www.R-project.org). Associations between genetic markers and phenotypes were tested using the Cochran–Armitage trend test.
Results
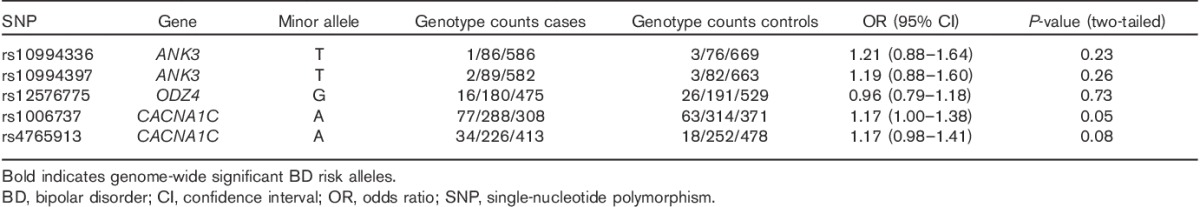
Rs1006737 in CACNA1C showed a nominally significant association with BPD in the total sample (P=0.0498). However, this result did not withstand correction for multiple testing (for details, see Table 1). Sex-specific analysis showed that this result was driven by an association in the female subsample (P=0.01). No association was observed between rs1006737 and BPD in the male subsample (P=0.39) (see SDI, Table S2a and S2b, Supplemental digital content 1, http://links.lww.com/PG/A115). For the remaining four SNPs of interest, no significant association with BPD was observed.
Table 1.
Allele frequencies and association results for all five SNPs investigated
Discussion
To investigate the hypothesis that BPD and BD share genetic variation, the present case–control study investigated whether five genome-wide significant risk variants identified for BD were associated with BPD. The analyses identified a nominally significant association between BPD and rs1006737 in CACNA1C (P=0.0498). In both disorders, the risk of disease was conferred by the A allele. No association with BPD was observed for the remaining four variants (Table 1). Sex-specific analysis of the SNPs showed that the rs1006737 signal was present in women (P=0.01), but not in men (P=0.39). A further SNP in CACNA1C (rs4765913) showed a significant association only in women (P=0.01) (Table S2a/b SDI, Supplemental digital content 1, http://links.lww.com/PG/A115). However, this variant is in high linkage disequilibrium with rs1006737 (D′=0.9), and thus this cannot be considered an independent finding.
Interestingly, previous studies have identified sex-specific associations between CACNA1C and BD. In particular, an association study of a combined BD and major depression dataset in 3,800 patients reported an association with the A allele of rs1006737 only in women (P=0.025) (Dao et al., 2010). Furthermore, sex-specific effects were found for personality traits (Strohmaier et al., 2013). Here, however, higher emotional lability and lower resilience were associated with the A allele in men and the G alleles in women, respectively (Strohmaier et al., 2013). This seemingly reversed allele effect is a well-known phenomenon encountered in other complex disorders as well (Lin et al., 2007). For a detailed discussion on this topic, see the study by Strohmaier et al. (2013).
Besides BD, CACNA1C has also been associated with major depression and schizophrenia (Green et al., 2010; Liu et al., 2011). This suggests that CACNA1C plays a general role in the pathomechanisms that underlie psychiatric disease. Imaging genetic studies have shown that rs1006737 also exerts pleiotropic effects on particular brain functions, such as the regulation of emotion and memory and attention networks, and that it affects different brain regions, including the amygdala, the hippocampus, and the ventrolateral prefrontal cortex. Alterations in these brain functions and regions have also been found in BPD, and several authors have proposed these alterations as pathogenetic models for BPD (Mauchnik and Schmahl, 2010; Krause-Utz et al., 2012; O’Neill and Frodl, 2012).
The present finding must be considered preliminary as it was only nominally significant and did not withstand correction for multiple testing. Furthermore, although our cohort represented the largest BPD cohort in published genetic studies to date, the sample size was limited compared with that required for successful association studies of other complex disorders (Visscher et al. 2012).
Novel statistical approaches now allow the analysis of an overlap in common genetic variation between diagnostic categories, and this approach has already been used for other major psychiatric diseases. For example, a meta-analysis of GWAS data for five psychiatric disorders showed a large genetic overlap (Lee et al., 2013). A plausible assumption therefore is that future studies of the GWAS data of large samples of BPD patients will provide further insights into its etiology and the genetic overlap between BPD and BD.
Conclusion
This is the first report of an association between a BD risk gene and BPD where selection was not based on a priori hypotheses about its function, but on an unbiased hypothesis-free screening of the genome. Genome-wide association data of large samples of BPD are warranted and will eventually identify new risk genes and the overlap between BPD and BD if it exists.
Supplementary Material
Acknowledgements
The authors thank Slavica Radosavljevic-Belic and Christine Hohmeyer for their expert technical laboratory work.
M.M.N., S.C., and M.R. are recipients of grants from the German Federal Ministry of Education and Research (BMBF) within the context of the Integrated Genome Research Network (IG) MooDS (Systematic Investigation of the Molecular Causes of Major Mood Disorders and Schizophrenia; grant 01GS08144 to M.M.N. and S.C.; grant 01GS08147 to M.R.).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Stephanie H. Witt and Nikolaus Kleindienst contributed equally to the writing of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.psychgenetics.com).
References
- Akiskal HS. (2004). Demystifying borderline personality: critique of the concept and unorthodox reflections on its natural kinship with the bipolar spectrum. Acta Psychiatr Scand 110:401–407. [DOI] [PubMed] [Google Scholar]
- Calati R, Gressier F, Balestri M, Serretti A. (2013). Genetic modulation of borderline personality disorder: systematic review and meta-analysis. J Psychiatr Res 47:1275–1287. [DOI] [PubMed] [Google Scholar]
- Coulston CM, Tanious M, Mulder RT, Porter RJ, Malhi GS. (2012). Bordering on bipolar: the overlap between borderline personality and bipolarity. Aust N Z J Psychiatry 46:506–521. [DOI] [PubMed] [Google Scholar]
- Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, et al. Bipolar Genome Study (BiGS) Consortium. (2010). Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry 68:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Willemsen G, Derom CA, Trull TJ, Martin NG, Boomsma DI. (2009). Familial resemblance of borderline personality disorder features: genetic or cultural transmission? PLoS One 4:e5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. (2008). Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 40:1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, et al. Wellcome Trust Case Control Consortium. (2010). The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Utz A, Oei NY, Niedtfeld I, Bohus M, Spinhoven P, Schmahl C, Elzinga BM. (2012). Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol Med 42:2181–2192. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Cross-Disorder Group of the Psychiatric Genomics Consortium; International Inflammatory Bowel Disease Genetics Consortium (IIBDGC). (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 45:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. (2004). Borderline personality disorder. Lancet 364:453–461. [DOI] [PubMed] [Google Scholar]
- Lin PI, Vance JM, Pericak-Vance MA, Martin ER. (2007). No gene is an island: the flip-flop phenomenon. Am J Hum Genet 80:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, et al. Wellcome Trust Case-Control Consortium. (2011). Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry 16:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loranger AW, Oldham JM, Tulis EH. (1982). Familial transmission of DSM-III borderline personality disorder. Arch Gen Psychiatry 39:795–799. [DOI] [PubMed] [Google Scholar]
- Mauchnik J, Schmahl C. (2010). The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep 12:46–55. [DOI] [PubMed] [Google Scholar]
- O’Neill A, Frodl T. (2012). Brain structure and function in borderline personality disorder. Brain Struct Funct 217:767–782. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Jonas JM, Hudson JI, Cohen BM, Gunderson JG. (1983). The validity of DSM-III borderline personality disorder. A phenomenologic, family history, treatment response, and long-term follow-up study. Arch Gen Psychiatry 40:23–30. [DOI] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group. (2011). Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 43:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohmaier J, Amelang M, Hothorn LA, Witt SH, Nieratschker V, Gerhard D, et al. (2013). The psychiatric vulnerability gene CACNA1C and its sex-specific relationship with personality traits, resilience factors and depressive symptoms in the general population. Mol Psychiatry 18:607–613. [DOI] [PubMed] [Google Scholar]
- Torgersen S, Lygren S, Oien PA, Skre I, Onstad S, Edvardsen J, et al. (2000). A twin study of personality disorders. Compr Psychiatry 41:416–425. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Brown MA, McCarthy MI, Yang J. (2012). Five years of GWAS discovery. Am J Hum Genet 90:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Morgan TA. (2013). Problematic boundaries in the diagnosis of bipolar disorder: the interface with borderline personality disorder. Curr Psychiatry Rep 15:422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


