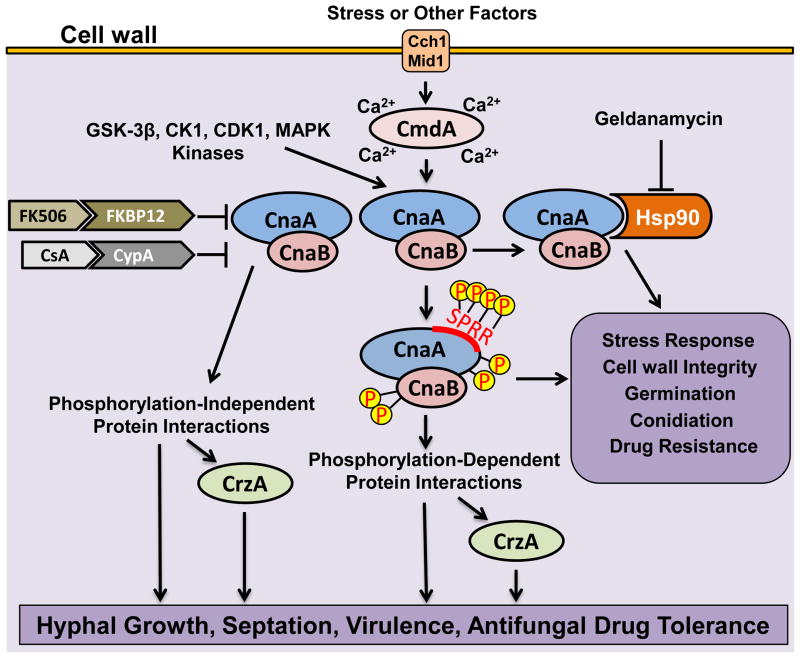
Figure 1.
Novel aspects of calcineurin regulation in A. fumigatus. Calcineurin, a heterodimer comprising of the catalytic subunit (CnaA) and the regulatory subunit (CnaB), is activated by Ca2+-calmodulin (CmdA) in response to increase in intracellular Ca2+ by the activity of calcium channels (Cch1/Mid1) and other intracellular stores (not shown). Calcineurin function is inhibited by the binding of the immunophilin-immunosuppresant complexes (FK506-FKBP12 and CsA-CypA). CnaA is phosphorylated at four serine residues in the Serine Proline Rich Region (SPRR) and also at two serine residues in the C-terminus, and two serine residues in the N-terminus of CnaB by the activity of kinases (GSK-3β, CK1, CDK1, MAPK). The phosphorylated calcineurin complex may bind to other substrates and regulate a subset of cellular functions through the transcription factor CrzA or independent of CrzA. Similarly, the unphosphorylated calcineurin complex may also exist and interact with substrates in a phosphorylation-independent manner to regulate other subsets of cellular functions in a CrzA-dependent or independent manner. Calcineurin also orchestrates its function through interaction with the heat shock protein Hsp90, which is a target of geldanamycin. The activated calcineurin-Hsp90 complex is involved in the regulation of stress response, cell wall integrity, growth and drug resistance.