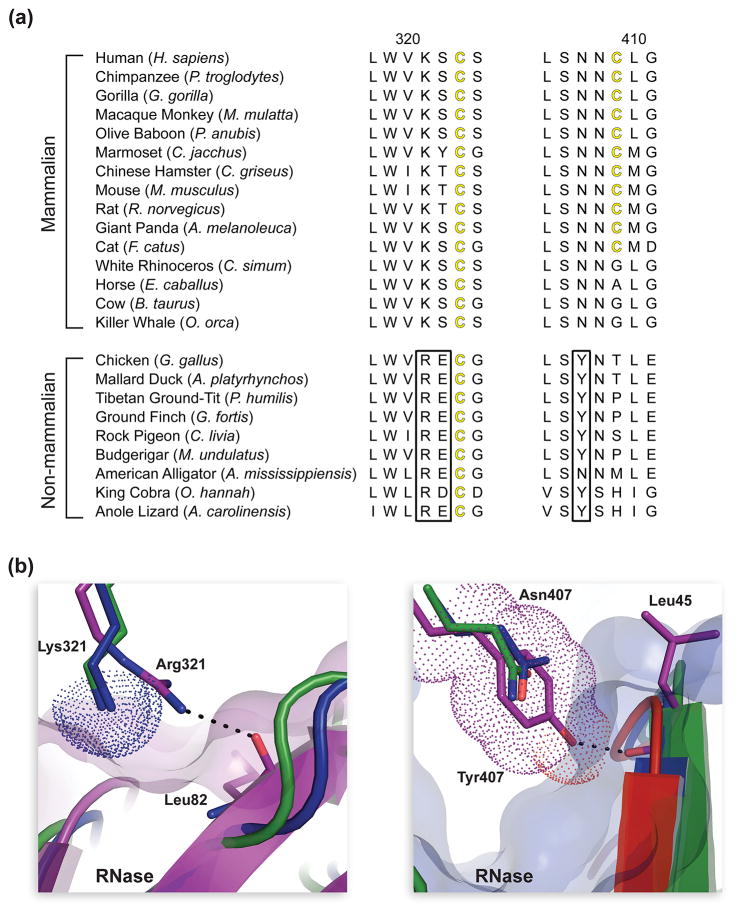
Fig. 5.
Evolution of residues at the interface of mammalian and non-mammalian RI·RNase complexes. (a)Two sections of amino-acid sequence alignment for homologous mammalian and non-mammalian RIs. Black boxes highlight residues conserved in non-mammalian RIs that are absent from mammalian homologs. (b) Two sections of tertiary structural alignment of the mouse (green), bovine (blue), and chicken (purple) RI·RNase complexes to illustrate the affect of amino-acid substitutions at position 321 and 407 of RI. The hydrogen bond of Arg321 in chicken RI with the main-chain oxygen of Leu86 in chicken RNase is not present in mammalian RI·RNase complexes, which contain a lysine residue at this position in RI. Lys321 (dotted surfaces) of cow and mouse RI could clash with bound chicken RNase (purple transparent surface). The hydrogen bond of Tyr407 in chicken RI with the main-chain oxygen of Leu43 in chicken RNase is not present in mammalian RI·RNase complexes, which contain an asparagine residue at this position in RI. Tyr407 (dotted surface) of chicken RI could clash with bound bovine RNase (blue transparent surface).