Abstract
Internal phosphorus (P) mobilisation from aquatic sediments is an important process adding to eutrophication problems in wetlands. Salinisation, a fast growing global problem, is thought to affect P behaviour. Although several studies have addressed the effects of salinisation, interactions between salinity changes and nutrient cycling in freshwater systems are not fully understood. To tackle eutrophication, a clear understanding of the interacting effects of sediment characteristics and surface water quality is vital. In the present study, P release from two eutrophic sediments, both characterized by high pore water P and very low pore water iron (Fe2+) concentrations, was studied in a long-term aquarium experiment, using three salinity levels. Sediment P release was expected to be mainly driven by diffusion, due to the eutrophic conditions and low iron availability. Unexpectedly, this only seemed to be the driving mechanism in the short term (0–10 weeks). In the long term (>80 weeks), P mobilisation was absent in most treatments. This can most likely be explained by the oxidation of the sediment-water interface where Fe2+ immobilises P, even though it is commonly assumed that free Fe2+ concentrations need to be higher for this. Therefore, a controlling mechanism is suggested in which the partial oxidation of iron-sulphides in the sediment plays a key role, releasing extra Fe2+ at the sediment-water interface. Although salinisation was shown to lower short-term P mobilisation as a result of increased calcium concentrations, it may increase long-term P mobilisation by the interactions between sulphate reduction and oxygen availability. Our study showed time-dependent responses of sediment P mobilisation in relation to salinity, suggesting that sulphur plays an important role in the release of P from FeSx-rich sediments, its biogeochemical effect depending on the availability of Fe2+ and O2.
Introduction
The eutrophication of surface waters is an urgent problem worldwide [1]. Increased P concentrations have led to a strong decline of the biodiversity in freshwater wetlands, due to the resulting dominance of highly competitive macrophytes, and of algae and cyanobacteria, monopolising light [1]–[3]. Salinisation of freshwater systems has received increasing attention, especially in relation to climate change and sea level rise [4]. With increasing salinity, higher P concentrations are often found in surface waters (e.g. [5]–[8]), which may affect P cycling in freshwater systems. Therefore, salinisation is expected to enhance eutrophication in coastal, freshwater wetlands, leading to water quality deterioration and loss of biodiversity.
Internal mobilisation of P from eutrophic aquatic sediments is an important process adding to eutrophication problems in wetlands [9]–[12]. The classic theoretical framework suggests that sufficiently high oxygen (O2) concentrations in the surface water can prevent P release from the sediment [13]–[14]. According to this, the oxidation of dissolved iron (Fe2+) in the sediment will result in the formation of iron oxides and hydroxides (Fe(OH)x) at the sediment surface, effectively binding P and thereby preventing its release to the surface water. Under anaerobic conditions, these ferric compounds will be mobilised by Fe-reducing bacteria, and part of the P is released to the surface water.
Besides anaerobic conditions, increased sulphate (SO4 2-) reduction rates are also known to be able to increase P mobilisation by decoupling Fe - P interactions at the sediment-water interface [12], [15]–[19]. Sulphide (S2-) binds efficiently to dissolved Fe2+ in sediment pore water, and most Fe2+ can become bound as iron sulphides (FeSx) in the sediment, strongly decreasing Fe2+ sediment pore water concentrations [18], [19]. Geurts et al. [19] found that, in aerobic surface waters, P mobilisation from sediments with low pore water Fe:P ratios (<1 mol mol-1) was a linear function of sediment pore water P concentrations. As a result, one would expect a release of P irrespective of the O2 concentration in the surface water of SO4 2- enriched wetlands [10], [12], [18], [20]. In addition, dissolved P concentrations might further increase due to the enhanced anaerobic breakdown of organic matter linked to SO4 2- reduction and concomitant mineralisation of P [2], [12], [19].
Salinisation of freshwater systems can enhance SO4 2- reduction rates due to a higher SO4 2- availability [21], which may strongly affect P mobilisation as described above. Moreover, increasing Cl- and SO4 2- concentrations might enhance P release from sediments by competition for anion binding sites [10], [22]. At the same time, an increase in salinity also leads to increased Ca2+ concentrations [21], which may result in the immobilisation of P by co-precipitation with Ca2+ and calcium carbonate (CaCO3) [4], [9], [23]. Salinity changes affect a suite of biogeochemical processes in freshwater systems, where the net effect on P mobilisation is the combined result of these processes. Moreover, a time-dependent shift in dominance of each process on P release can be expected [18]. Most studies regarding P release focus on relative short-term effects ranging from one day to 90 days [6], [19], [20], [24], while long-term experiments are mostly lacking. In this paper we explore the time-dependent release of P from eutrophic sediments under different salinities, which is highly relevant regarding the worldwide interest in salinisation effects on freshwater wetland functioning.
To test time-dependent interactions between salinisation and P mobilisation, a controlled aquarium experiment was set up that lasted two years. Two FeSx-rich sediments from a coastal freshwater wetland were subjected to three naturally occurring water types characterised by different salinities. Pore waters of the peat sediments were typically rich in P and S, and very poor in Fe, and the low total Fe:S ratios in the sediment suggested that most Fe was bound to reduced S [2]. In such sediments, a very high release of P from the sediment to the surface water can be expected, predominantly depending on pore water P concentrations [10], [12], [18]–[20]. By monitoring biogeochemical changes in porewater and surface water under controlled conditions, we try to reveal how salinity affects short-term and long-term P release, in these type of sediments common for coastal wetlands.
Materials and Methods
2.1 Sampling area
In this study, peat sediments were used from the coastal lowland fen area Wormer- and Jisperveld (52° 30′ 42.7644″; 4° 52′ 27.3756″) in the Netherlands. Due to historic intrusion of brackish water, peat rich in minerals such as S, Ca and Fe has accumulated in this area. After more than 50 years of desalinisation resulting from altered hydrological conditions, it gradually became a freshwater system. The peatland comprises ca. 500 ha of open water and ca. 1660 ha of peat meadows, predominantly used for agricultural purposes and partly for nature conservation. Drainage is a standard procedure in this area, leading to peat decomposition and land subsidence. As a result, risks of flooding events and salinisation are increasing in this freshwater peatland.
2.2 Experimental design
On 18 March 2008, two types of submerged peat sediment were collected from a ditch at a depth of 0–20 cm (ca. 25 L in total), using a sediment multi sampler (Eijkelkamp Agrisearch Equipment). Although both sediments were relatively rich in organic S and P, they differed in P availability (sediment characteristics are given in Table 1). To minimise O2 intrusion, the sediments were stored anaerobically at 4°C in large, closed containers. The next day, 12 glass cylinders (diameter 15 cm, height 60 cm) were filled with 15 cm of sediment A and another 12 cylinders with 15 cm of sediment B. Next 40 cm of water was carefully poured on top of the sediments, avoiding re-suspension of sediment particles. Artificially composed surface water, based on site conditions (control treatment, Table 2), was used for all sediments during an acclimatisation period of 4 weeks. The experiment was carried out in the dark at a constant and environmentally relevant temperature of 15°C. To allow oxygen diffusion to the surface water, an open cylinder system was used.
Table 1. Characteristics of the two sediments used.
| Organic | Bulk | Total amounts bound to sediment | |||||||||
| Sediment | content | Density | Total - P | Org - P | Inorg - P | Total - Al | Total - Ca | Total - S | Total - Fe | Fe:S ratio | |
| % | kg DW L−1 FW | mmol L−1 FW | mmol L−1 FW | mmol L−1 FW | mmol L−1 FW | mmol L−1 FW | mmol L−1 FW | mmol L−1 FW | mol mol−1 | ||
| A | Mean | 59.7 | 0.17 | 2.9a | 1.8 | 1.2a | 44.5 | 54.9 | 99.3 | 33.1 | 0.33a |
| SEM | 4.8 | 0.03 | 0.4 | 0.3 | 0.1 | 12.6 | 5.4 | 8.1 | 3.9 | 0.024 | |
| B | Mean | 59.6 | 0.13 | 4.7b | 2.4 | 2.3b | 43.4 | 44.0 | 83.6 | 38.3 | 0.46b |
| SEM | 7.4 | 0.02 | 0.3 | 0.1 | 0.1 | 4.4 | 0.8 | 4.7 | 2.3 | 0.001 | |
Significant differences between the sediment types are indicated by different letters.
Table 2. Chemical composition of the surface water used for the different treatments (low, normal or high salinity).
| Low salinity | Normal salinity | High salinity | |
| Rain water | Fresh water | Brackish water | |
| Element | µmol L−1 | µmol L−1 | µmol L−1 |
| Na+ | 100 | 7000 | 85000 |
| Cl- | 100 | 7000 | 85000 |
| SO4 2- | 5 | 1500 | 5500 |
| K+ | 30 | 500 | 1000 |
| Ca2+ | 10 | 2000 | 2500 |
| Mg2+ | 10 | 1250 | 3750 |
| HCO3 - | 0 | 4000 | 4000 |
| NO3 - | 50 | 50 | 50 |
| NH4 + | 50 | 50 | 50 |
After this acclimatisation period, three different surface water types were applied as salinity treatments: rainwater (low salinity; 100 µmol Cl L−1), brackish water (high salinity; 85 mmol Cl L−1) and freshwater (control; 7 mmol Cl L−1). All treatments were artificially composed, based on field measurements (Table 2). Control water simulated water quality in the current conditions that exist in the wetland, brackish water composition was based on the historic conditions reported by Reigersman in 1946 [25]. Rainwater quality equalled the chemical composition of atmospheric deposition as measured in the Netherlands [26]. No P was added in order to be able to estimate the release of P from the sediment. For each treatment and sediment type, 4 replicates were used (24 cylinders in total).
Treatment solutions were stored in polyethylene containers (10 L), from which they were pumped into the cylinders using Masterflex L/S multichannel pumps (model 7535-08). The treatments were started by replacing the control water with the appropriate treatment water during 4 weeks, to ensure that all treatment solutions were added properly. Directly after treatment addition (week 10), stagnant conditions were created in order to measure short-term effects of P and S release from the sediment. Short-term mobilisation rates were calculated from the linear increase of the surface water P and S concentrations (0–10 weeks of the stagnant period). After a stagnant period of 26 weeks, pumps were running with a hydraulic retention time of 25 weeks for the treatment solutions during 48 weeks in order to maintain the appropriate treatment conditions. To measure long-term effects of salinity changes on the release of P and S from the sediment, pumps were stopped again (week 81) to create another stagnant period for 32 weeks. Long-term mobilisation rates were again calculated from the linear increase of the S and P concentrations in the surface water during this stagnant period.
Intact peat cores from the same location as the main experiment were collected separately, to test the effects of aerobic versus anaerobic conditions of the surface water on P release. Water and sediment oxygen (O2) profiles were measured, using a fixed fiber optical oxygen microsensor (optode) in combination with a Microx TX3 transmitter (PreSens Precision Sensing GmbH). The peat sediment cores were monitored during 18 weeks of either aerobic conditions similar to those of the main experiment, or anaerobic conditions by gently supplying N2 to the surface water. During both aerobic and anaerobic conditions, P mobilisation rates were calculated from the linear increase in surface water P concentrations.
2.3 Chemical analyses
To monitor water quality, samples of surface water and pore water were collected every 2 months and analysed during the experiment. Pore water was collected anaerobically, using 30 mL vacuum bottles connected to Rhizon SMS-10 cm samplers that were fixed in the upper 10 cm of the sediment (Eijkelkamp Agrisearch Equipment). Disturbance of the sediment and water was minimised by the low frequency of sampling and small sample sizes (max. 25 mL). Sulphide concentrations were determined directly after the collection by fixing 10.5 mL pore water with 10.5 mL Sulphide Anti Oxidant Buffer (SAOB), and using an Orion sulphide-electrode and a Consort Ion meter (type C830) [27]. The pH and alkalinity of all samples were measured within 24 hours after sampling, using a combined pH electrode (Radiometer) in combination with a TIM840 pH meter and a Titration Manager Titralab Autoburette. Dissolved total inorganic carbon (TIC) was measured within 24 hours after sampling by injecting 0.2 mL pore water or surface water in a closed chamber containing 0.2 M H3PO4 solution, converting all dissolved TIC into CO2. A continues gas flow (N2) directly transports the CO2 to an ABB Advance optima Infrared Gas Analyzer (IRGA) to measure total inorganic C concentrations. A calibration curve was made by injecting different volumes (0.1–1.0 mL) of 1.25 mM HCO3 - solution. Prior to storage at 4°C until elemental analysis, 0.1 mL HNO3 - (65%) was added to 10 mL of each sample to prevent metal precipitation. Concentrations of dissolved Ca, Fe, P, S, and Al in these stored samples were measured using an Inductively Coupled Plasma Spectrophotometer (ICP IRIS Intrepid ΙΙ XDL; Thermo Electron Corporation). Due to the anaerobic sampling of pore water, measured Fe predominantly consisted of dissolved Fe2+ rather than far less mobile Fe3+. The remaining samples were stored at −20°C in order to determine the following ion concentrations colourimetrically on Auto Analyzer 3 systems (Bran and Luebbe): NO3 - [28], NH4 + [29], ortho-PO4 3- [30] and Cl- [31]. Na+ and K+ were determined with a Technicon Flame Photometer IV Control (Technicon Corporation).
For both sediments gravimetric water contents were determined by drying for 48 h at 70°C. Organic matter contents were estimated by loss on ignition for 4 h at 550°C. A homogenized portion of 200 mg dry sediment was digested in 5 mL HNO3 (65%) and 2 mL H2O2 (30%), using an Ethos 1 Advanced microwave digestion system (Milestone Inc.). Digestates were diluted and analysed by ICP as described above. In order to distinguish between the organic and inorganic P fraction, a P-fractionation procedure was carried out adapted after Golterman [32].
2.4 Statistical analyses
For statistical analysis, SPSS Statistics for Windows (Version 21.0. IBM Corp. Armonk, NY; 2012) was used. To test for differences among treatments in sediment analyses (single measurements) or differences in calculated mobilisation rates, the General Linear Model (GLM) univariate procedure combined with Tukey's-b post-hoc test was used.
To test for significant differences among treatments in repeated measurements, a GLM mixed model procedure was used. When significant differences between the two sediments were found, using a 2-way GLM mixed model with treatment as fixed factor, sediment as random factor and time as repeated measures, both sediments were analysed separately. In this separate model for sediments, time was used as repeated measures and treatment as fixed factor, with AR(1) heterogeneous as the covariance type. A Bonferroni post-hoc test was used to test for differences between treatments.
2.5 Ethics statement
This study was part of the National Research Programme ‘Wormer- en Jisperwater’, funded by the Dutch Ministry of Agriculture, Nature and Food Quality (LNV), within the framework of ‘Nota Ruimte’. The water management authority ‘Hoogheemraadschap Hollands Noorderkwartier’ facilitated this programme and the nature management authority ‘Natuurmonumenten’ gave permission to take samples in their reserve.
Results
3.1 Pore water chemistry
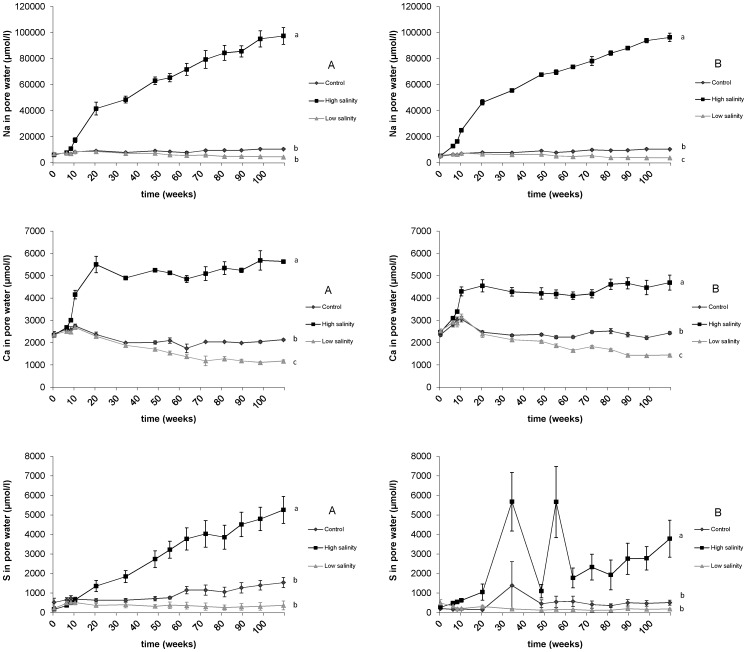
As expected, pore water chemistry was strongly affected by changes in surface water salinity (Fig. 1). Under brackish conditions, Na+ showed a highly significant (p<0.005) gradual increase in the pore water over time. For the low salinity and control treatment, no significant changes in pore water Na+ concentrations occurred in sediment A, while Na+ concentrations showed a significant decrease (p<0.05) over time at a low salinity in sediment B. An interaction between treatment and sediment type was found for both Na+ and S concentrations, which means that the treatments had a significant, but different, effect on the two sediments. Pore water S concentrations also showed a highly significant (p<0.005) gradual increase at the high salinity treatment in both sediments (Fig. 1). At a low salinity, S concentrations remained at a steady level while the control treatment showed a small, but not significant, increase. Moreover, no clear differences in sulphide concentrations were found between sediments or treatments (average values ranged between 0–50 µmol L−1 for sediment A, and between 0–500 µmol L−1 for sediment B; data not shown).
Figure 1. Sodium (Na+), calcium (Ca2+) and sulphur (S) pore water concentrations (µmol L−1) in both sediments (A: left, B: right).
Significant differences between treatments are indicated with different letters.
As a result of a higher salinity, Ca2+ was mobilised in the sediment, as shown by significantly (p<0.005) increased pore water Ca2+ concentrations (Fig. 1). This increase in the pore water, well above the added concentration of 2500 µmol L−1 to the surface water, started directly after the onset of the high salinity treatment and Ca2+ concentrations remained at a steady high level during the course of the experiment.
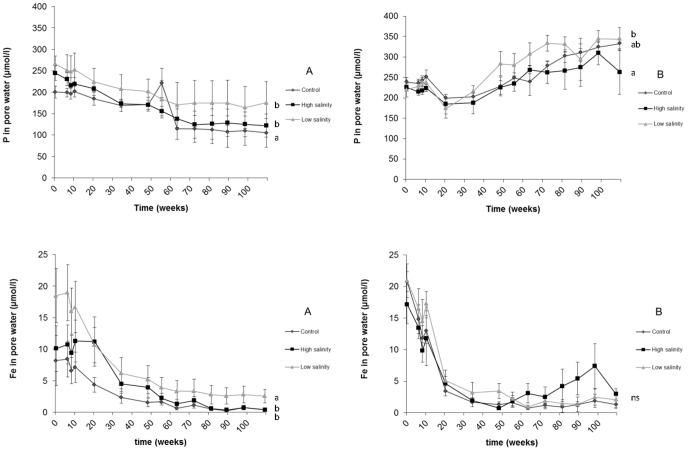
Dissolved Fe2+ concentrations were low and showed a gradual decrease for all treatments over time in both sediment types (Fig. 2). A significantly higher (p<0.005) Fe2+ concentration was found for the low salinity treatment at sediment A when compared to the control and higher salinity treatment. In contrast, no differences in pore water Fe2+ concentrations between treatments were found for sediment B. Pore water HCO3 - concentrations were significantly higher (p<0.05) under brackish conditions at sediment B, while no differences were found between treatments at sediment A (data not shown).
Figure 2. Phosphorus (P) and iron (Fe2+) concentrations (µmol L−1) in pore water of both sediments (A: left, B: right).
Significant differences between treatments are indicated with different letters.
Pore water P concentrations showed a gradual decrease at sediment A for all treatments. Moreover, a significantly (p<0.05) stronger decrease of P in pore water was found for the control treatment, compared to the high and low salinity treatment at sediment A. In strong contrast, P concentrations showed a gradual increase in the pore water of sediment B for all salinity treatments (Fig. 2), with the significantly (p<0.05) lowest P concentrations in the high salinity treatment.
3.2 Surface water chemistry
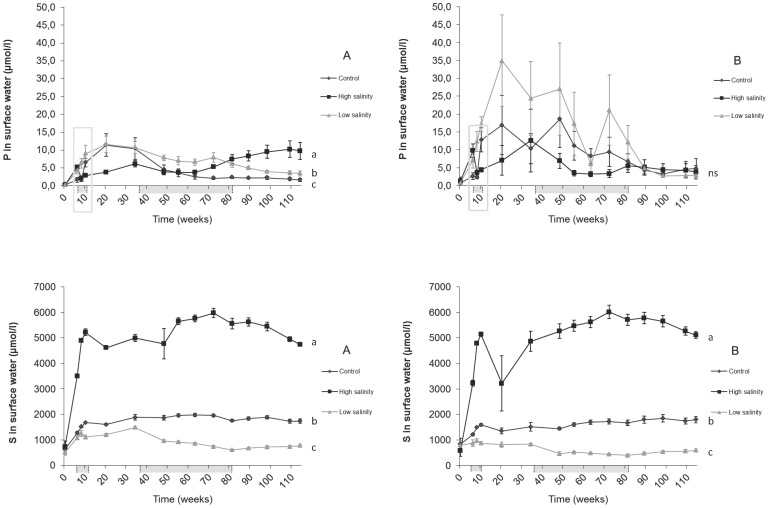
A higher salinity led to gradually increased Na+ and S concentrations in the surface water, and showed significant (p<0.005) differences among all treatments, which eventually equalled the concentrations added (S: Fig. 3; Na+: data not shown). For the low salinity treatment, however, S concentrations in the surface water reached much higher concentrations than the concentrations of the treatment water, which suggests S mobilisation from the sediment. These S mobilisation rates were calculated (Table 3) for both a short term, showing significantly (p<0.005) negative rates at a high salinity (high S consumption) for sediment A, and for a long term, still showing significantly (p<0.005) negative S mobilisation rates at a high salinity in both sediments. In the surface water, Ca2+ concentrations also increased and differed significantly (p<0.005) among all treatments for both sediments (data not shown). However, both the low and high salinity treatment led to much higher concentrations than the added concentrations.
Figure 3. Phosphorus (P) and sulphur (S) concentrations (µmol L−1) in the surface water above both sediments (A: left, B: right).
Significant differences between treatments are indicated with different letters. Grey shadings under the x-axis indicate periods with through-flow (see Materials and Methods).
Table 3. P and S mobilisation rates (µmol m−2 day−1) during stagnant conditions in the short term (0–10 weeks) and in the long term (80–110 weeks).
| P mobilisation (µmol m−2 day−1) | S mobilisation (µmol m−2 day−1) | |||||
| short term | long term | short term | long term | |||
| A | High salinity | Mean | 6.8 | 4.1 a | −1916.4 a | −1447.5 a |
| SEM | 1.6 | 2.4 | 86.7 | 243.0 | ||
| Control | Mean | 37.7 | −1.1 ab | 140.9 b | −87.0 b | |
| SEM | 14.3 | 0.6 | 216.0 | 86.4 | ||
| Low salinity | Mean | 19.8 | −4.0 b | −6.5 b | 244.2 b | |
| SEM | 12.9 | 2.3 | 425.0 | 69.2 | ||
| B | High salinity | Mean | 16.3 | −2.9 | −8536.5 | −1121.7 a |
| SEM | 24.2 | 1.0 | 5474.0 | 141.3 | ||
| Control | Mean | 53.3 | −2.2 | −840.4 | 123.6 b | |
| SEM | 36.6 | 2.5 | 517.8 | 91.1 | ||
| Low salinity | Mean | 102.6 | −13.1 | −597.4 | 294.8 b | |
| SEM | 74.3 | 6.5 | 236.3 | 83.3 | ||
Significant differences between treatments are indicated by different letters.
In the low salinity and control treatment, P concentrations in the surface water increased directly after onset of the treatments (after 10 weeks; t = 0). For the high salinity treatments, P concentrations of the surface water showed a strong and significant (P<0.05) decrease immediately after the onset of the treatments (after 10 weeks; t = 0). After this temporary decrease, P concentrations started to increase gradually. As a result, significantly lower P concentrations (p<0.05) were found for the high salinity treatment compared to the low salinity treatment in both sediments at a short term (after 20 weeks; t = 10), and a trend was found when compared to the control treatment (p<0.1) at sediment A. When P mobilisation rates were calculated for the short term, however, no differences among salinity treatments were found (Table 3).
More than 80 weeks after the start of the experiment, P concentrations in the surface water above sediment A were significantly higher (p<0.01) at a high salinity (Fig. 3), which was totally opposite to the short-term effect. Calculated P mobilisation rates were also significantly higher (p<0.05) with a high salinity compared to a low salinity at sediment A. For the control and low salinity treatment, P concentrations in surface water remained low, or even showed a decrease in the long term. At sediment B, however, no change of P in the surface water was found for any of the salinity treatments. The long-term P mobilisation rates with a high salinity were similar to the short-term rates at sediment A, while no long-term P mobilisation was observed for the low salinity and control treatments.
3.3 Aerobic versus anaerobic surface water
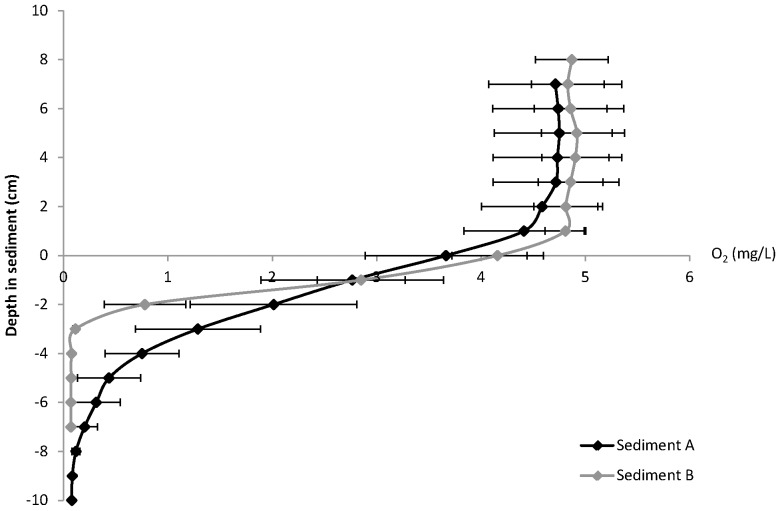
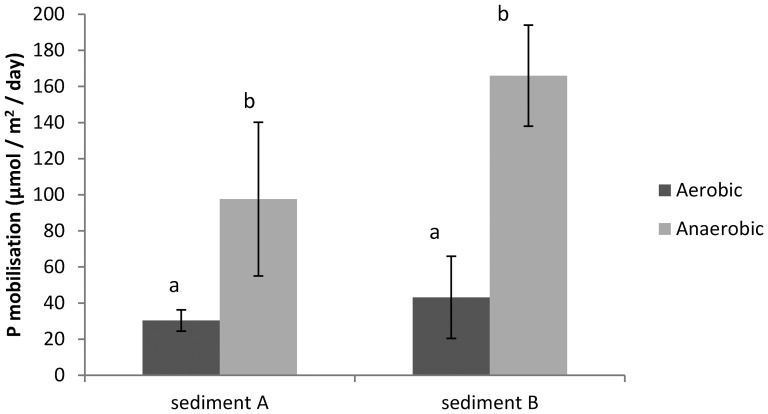
The O2 concentration profile (Fig. 4) shows that under aerobic conditions, O2 is still available in the sediment to an average depth of 7 mm (sediment A) and 3 mm (sediment B). The cores of both sediment A and B showed a significant (p<0.001) higher mobilisation rate of P during anaerobic conditions (Fig. 5). At sediment A, P mobilisation was on average 3 times higher during anaerobic conditions compared to aerobic conditions, while this was almost 4 times higher at sediment B. These aerobic mobilisation rates were well within range of the short-term mobilisation rates found in the main experiment (control treatment; Table 3).
Figure 4. Oxygen (O2) concentration (mg L−1) profile per mm of both sediments (A and B), at the sediment-water interface (indicated by vertical dotted line) during aerobic and anaerobic conditions.
Figure 5. P mobilisation rates (µmol m−2 day−1) during aerobic and anaerobic conditions for both sediment cores (A and B).
Significant differences between treatments are indicated with different letters.
Discussion
4.1 Short-term effects (0–10 weeks)
4.1.1 P mobilisation
In the short term, no differences in net mobilisation rates of P were found among the different treatments. During this first stagnant period, moderate P mobilisation rates of 7–103 µmol m−2 d−1 were found that fitted within the range of Geurts et al. [19], who found mobilisation rates of 10–150 µmol m−2 d−1 for sediments of which pore water Fe:P and total sediment Fe:S ratios were <1. Diffusion was most likely the main mechanism driving P release [19], [33], since the sediments used in this experiment were not subjected to bioturbation or resuspension [7], nor to a changed pH or temperature [24]. Moreover, both sediments were characterised by total Fe:S ratios below 0.5 (Table 1), which indicates that most Fe was bound to reduced S [20]. Indeed, dissolved pore water Fe2+ concentrations were low in this study (and ranged between 0–20 µmol L−1 for both sediments), and showed an even further decrease over time, resulting in very low pore water Fe:P ratios (<0.1) during the entire experimental period.
4.1.2 Salinity effects
Although increased salinity may lead to increased desorption of P from anion exchange sites [10], or by increased S2- production and enhanced mineralisation rates [2], [12], we did not find higher pore water P concentrations in the high salinity treatment. Instead, during the addition of the salinity treatments (between week 6 and 10), P concentrations in the surface water showed a short, strong drop for both sediments. This immediate drop of P observed upon a change of the surface water chemistry strongly points at a chemical, rather than a microbiological, explanation. It can most likely be explained by the co-precipitation of P with Ca2+ or CaCO3 at the sediment-water interface [4], [9], as Ca2+ concentrations directly and strongly increased in both surface and pore water upon the high salinity treatment (0–10 weeks). Accordingly, Suzumura et al. [7] found a fast chemical P (im)mobilisation response within minutes, due to adsorption-desorption processes after a changed salinity. Van Dijk et al. [34] found a similar immobilisation of P with increased salinity, explained by co-precipitation with Ca2+ in the sediment. Degassing of carbon dioxide (CO2) and possibly also the presence of microbial mats [35] may well have contributed to the precipitation of CaCO3 at the sediment surface, as HCO3 - concentrations were up to three times higher in pore water than in the surface water. After the initial drop of P, concentrations started to gradually increase, which shows that the short-term overall net P mobilisation to the surface water was higher than its immobilisation due to co-precipitation with Ca2-.
4.2 Long-term effects (1.5–2 years)
4.2.1 P mobilisation
In contrast to the short-term results, and rather unexpectedly for eutrophic sediments, P mobilisation to the surface water was absent in 5 out of 6 treatments in the longer term (after 80 weeks). This is remarkable, as a strong net diffusive P release in both sediments was expected given the very low pore water Fe2+ concentrations and the still very high pore water P concentrations [19]. Although a gradual decrease of P in the pore water of sediment A was observed, concentrations still remained sufficiently high for diffusive P release (>100 µmol L−1) [19], [20]. Sediment B even showed a gradual increase of pore water P concentrations during the experiment, without any increase of the P mobilisation to the surface water. Such results can only be explained by assuming that processes preventing net P release at the sediment-water interface become active in the long term, at least under the conditions that were created during our experiment. Possible explanations for this phenomenon are: (1) precipitation of P with Fe3+ or Fe(OH)x by the oxidation of the sediment surface [13], [14], (2) storage of P by the microbial community at the sediment surface during aerobic conditions [36], [37], (3) precipitation of P with calcium-minerals [9], [23], although the latter would mainly be expected in the high salinity treatment.
An explanation for the lack of P release in the long term might be the uptake of P by microbial mats growing on top of the sediment [35]–[37]. These mats can develop over time and might also benefit from stable sediment conditions that developed in the experimental set-up. However, our experiment was carried out in the dark, excluding photosynthetically active organisms, and no visible signs of such mats were observed. Nevertheless, the potential role of microbial sequestration of P on the long term cannot be ruled out.
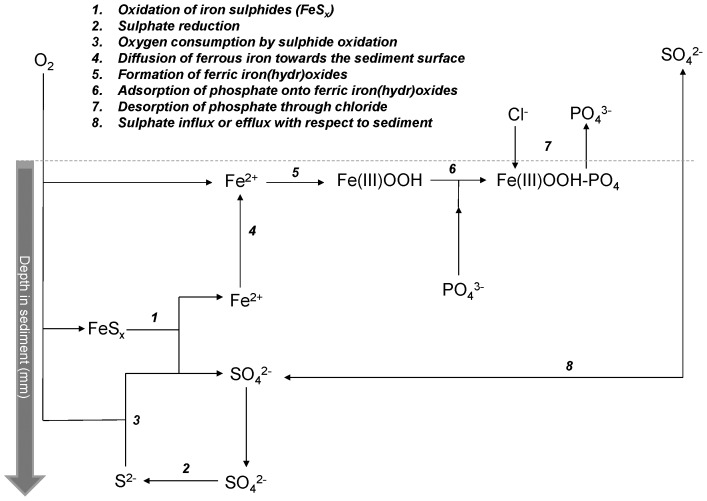
Most likely Fe redox cycling played a dominant role in the absence of P mobilisation, as was also indicated by the strongly increased P release under anaerobic conditions compared to aerobic conditions (Fig. 5). It has been demonstrated that diffusive P release should be prevented under aerobic conditions if pore water Fe:P ratios are relatively high (at least >1) [19], [38], [39]. In our sediments, however, pore water Fe:P ratios were very unfavourable. Nevertheless, oxidation processes might be able to mobilise Fe2+ from FeSx at a spatial micro-scale in the sediment surface at relatively low O2 levels [40], catalysed by S oxidising microbes [41]. Our O2 profiles showed that O2 was available in the surface water and in the top millimetres of the sediment. The observed high S mobilisation rates in the low salinity treatment, where no S was added, indeed showed that SO4 2- is being mobilised from the sediment by the oxidation of FeSx. Simultaneously, Fe2+ thus becomes available to be oxidised [40], and is able to sequester dissolved P. So the intrusion of O2 in reduced sediments may mobilise S bound Fe at a millimetre spatial scale, providing dissolved Fe2+ for the formation of ferric Fe(OH)x at the sediment surface (Fig. 6). This mechanism may very well explain the unexpected lack of P release from the sediments in the long term under aerobic conditions.
Figure 6. Schematic overview of the proposed mechanism, showing key processes in the upper millimetres of the S-rich, peat sediments involved in P mobilisation.
Salinisation leads to an increased SO4 2- influx, affecting Fe diffusion to the sediment surface, enabling increased P mobilisation in the longer term.
Our experimental set-up, without sediment disturbance and with relatively low biochemical O2 demand (BOD) due to the absence of fresh organic matter input, will certainly have contributed to the long-term outcome of the experiment. Nevertheless, it seems plausible that it took a relatively long time before the sediment surface became sufficiently oxidised, or before the microbial population was sufficiently developed, to completely prevent P mobilisation in the experiment. These results in the longer term may represent field situations with stable non-bioturbated FeSx-rich sediments or sediments with stagnant, hypolimnetic water. During anaerobic conditions, P mobilisation was strongly enhanced (Fig. 5), which clearly highlights the importance of O2 availability to prevent P release. Field experiments are, therefore, necessary to validate our experimental results and suggested mechanism for the lack of P release from S-rich aquatic sediments.
4.2.2 Salinity effects
For the high salinity treatment, one of the sediments showed an increase of the surface water P concentration also in the long term (80 weeks). In saline or estuarine systems, P is often found to be easily released from soil particles [5], [7], and dissolved P concentrations are usually higher with increasing salinity [6], [8], [39]. At a high salinity, SO4 2- concentrations increased in both surface water and pore water and a considerable part may be reduced deeper in the sediment, since it was not released to the surface water (Fig. 6). Produced S2- will react with O2 and interfere with the oxidation of FeSx, or again immobilise Fe2+. As expected, the net mobilisation of Fe2+ will be less, leading to insufficient formation of ferric Fe(OH)x to prevent the release of P to the surface water [18], [19]. This decoupling of the Fe and P cycle [10] at a micro-scale diminishes the P-binding capacity at the water-sediment interface. In sediment A, O2 penetrated deeper into the sediment, suggesting that less O2 was consumed, less FeSx was oxidised, and less Fe2+ was mobilised. This may partly explain the long-term release of P from sediment A in the high salinity treatment. Desorption of P from ferric Fe(OH)x due to the high Cl concentrations [10], [22] might have increased this effect.
4.3 Implications for water management
Although the mobilisation of P from the S-rich and relatively Fe-poor sediments (typical for coastal wetlands) was mainly driven by diffusion, the build-up of a stable oxidised sediment surface may have prevented the release of P under the experimental conditions. We hypothesise that the oxidation of FeSx in the sediment surface delivers the Fe2+ necessary for the precipitation of P at the sediment-water interface (Fig. 6). Disturbance of the sediment-water interface due to wind, ebullition of gases from the sediment, and bioturbation can, however, prevent this build-up of a protective Fe-rich sediment surface and potentially increase the release of P [9], [33]. Although such processes might also mix the sediment surface with O2 and have an opposite effect. Moreover, our results indicate that an increased salinity may lead to a long-term P release, probably by interfering with the Fe2+ mobilisation due to increased SO4 2- reduction rates in the anaerobic sediment. They also point out that sediments may react differently upon increased salinity. Therefore, O2 and BOD, but also the actual concentration of SO4 2- play a key role in the mobilisation of P from FeSx-rich sediments. This might have important implications for water management and nature management of eutrophic peatlands in relation to salinisation.
More research, especially field measurements, is necessary to further confirm the experimental results we found for these FeSx-rich sediments. Our experiment was carried out at 15°C and without the continuous input of reactive organic material. Warmer conditions, e.g. during warm episodes in summer will lead to increased mineralisation rates, and also to higher O2 consumption rates and lower solubility of O2. Especially when there is a high input of reactive organic matter, this will lead to strongly decreased O2 concentrations in the surface water, which may prevent adequate oxidation of the sediment surface. Under such conditions this biogeochemical mechanism is expected to fail, leading to strong P mobilisation from the sediment as was shown in this study and also found by Smolders et al. [12]. As a result, floating-leaved species, or floating beds of algae or cyanobacteria may develop, which will further decrease the O2 concentrations in the surface water and enhance sediment P mobilisation. This explains why FeSx-rich sediments that show very high dissolved P concentrations and low dissolved Fe2+ concentrations tend to show a high P release mainly in summer, which has important implications for water management.
Conclusions
Low pore water Fe:P ratios indicated a decoupling of the Fe and P cycle. Although these FeSx-rich sediments were expected to release significant amounts of P by diffusion, this only seemed to be the case in the short term under aerobic conditions.
Increased salinity led to co-precipitation of P with Ca2+ in the short term, lowering actual P concentrations. However, short-term P mobilisation rates were found to be similar for all treatments, regardless of salinity.
Our experimental results suggest that the classic theoretical framework of oxidative conditions in the surface water that prevent P release from the sediment, may also hold in sediments showing unfavourable total Fe:S ratios but high FeSx concentrations. In our FeSx-rich, eutrophic sediments, typical for coastal wetlands, O2 availability still seemed to be the most important determinant of sediment P release, at least under stable sediment conditions.
We suggest a controlling mechanism in which the partial oxidation of FeSx mobilises sufficient Fe2+ at micro-scale for the precipitation of P at the sediment-water interface.
Next to O2, SO4 2- plays a key role in P mobilisation, as high concentrations may counteract the oxidising effect by immobilising Fe2+. In the longer term, an increased salinity may, as a result, led to P mobilisation despite oxidation of the sediment surface.
Acknowledgments
We would like to thank Jeroen Graafland and Rick Kuiperij for their practical assistance in the field and chemical analyses, Jelle Eygensteyn, Paul van der Ven and Sebastian Krosse for their help with chemical analyses, and Leon van den Berg for his help with statistical analyses. We are grateful to the water management authority ‘Hoogheemraadschap Hollands Noorderkwartier’ for the facilitation of this programme and to the nature management authority ‘Natuurmonumenten’ for giving their kind permission to take samples in their reserve.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was part of the National Research Programme “Wormer- en Jisperwater,” funded by the Dutch Ministry of Agriculture, Nature and Food Quality (LNV), within the framework of “Nota Ruimte.” The water management authority “Hoogheemraadschap Hollands Noorderkwartier” facilitated this programme. The authors did not require further external funding source for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Smith VH (2003) Eutrophication of Freshwater and Coastal Marine Ecosystems. A Global Problem. Environ Science & Pollution Research 10 (2): 126–139. [DOI] [PubMed] [Google Scholar]
- 2. Lamers LPM, Falla S-J, Samborska EM, van Dulken IAR, van Hengstum G, et al. (2002) Factors Controlling the Extent of Eutrophication and Toxicity in Sulfate-Polluted Freshwater Wetlands. Limnology and Oceanography 47 (2): 585–593. [Google Scholar]
- 3. Geurts JJM, Sarneel JM, Willers BJC, Roelofs JGM, Verhoeven JTA, et al. (2009) Interacting effects of sulphate pollution, sulphide toxicity and eutrophication on vegetation development in fens: A mesocosm experiment. Environmental Pollution 157 (7): 2072–2081. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen DL, Brock MA, Rees GN, Baldwin DS (2003) Effects of increasing salinity on freshwater ecosystems in Australia. Australian Journal of Botany 51: 655–665. [Google Scholar]
- 5. Carpenter PD, Smith JD (1984) Effect of pH, iron and humic acid on the estuarine behaviour of phosphate. Environmental Technology Letters 6: 65–72. [Google Scholar]
- 6. Gunnars A, Blomqvist S (1997) Phosphate exchange across the sediment-water interface when shifting from anoxic to oxic conditions – an experimental comparison of freshwater and brackish-marine systems. Biogeochemistry 37: 203–226. [Google Scholar]
- 7. Suzumura M, Udea S, Sumi E (2000) Control of phosphate concentration through adsorption and desorption processes in groundwater and seawater mixing at sandy beaches in Tokyo Bay, Japan. Journal of Oceanography 56: 667–673. [Google Scholar]
- 8. Jordan TE, Cornwell JC, Boynton WR, Anderson JT (2008) Changes in phosphorus biogeochemistry along an estuarine salinity gradient: The iron conveyer belt. Limnology and Oceanography 53 (1): 172–184. [Google Scholar]
- 9. Boström B, Andersen JM, Fleisher S, Jansson M (1988) Exchange of phosphorus across the sediment-water interface. Hydrobiologia 170: 229–244. [Google Scholar]
- 10. Caraco NF, Cole JJ, Likens GE (1989) Evidence for sulphate-controlled phosphorus release from sediments of aquatic systems. Nature 341: 316–317. [Google Scholar]
- 11. Lamers LPM, Tomassen HBM, Roelofs JGM (1998) Sulfate-Induced Eutrophication and Phytotoxicity in Freshwater Wetlands. Environmental Science and Technology 32: 199–205. [Google Scholar]
- 12. Smolders AJP, Lamers LPM, Lucassen ECHET, van der Velde G, Roelofs JGM (2006) Internal eutrophication: How it works and what to do about it – a review. Chemistry and Ecology 22 (2): 93–111. [Google Scholar]
- 13. Einsele W (1936) Über die Beziehungen des Eisenkreislaufs zum Phosphatkreislauf im eutrophen See. Archiv für Hydrobiologie 29: 664–686. [Google Scholar]
- 14. Mortimer CH (1941, 1942) The exchange of dissolved substances between mud and water in lakes. J. Ecology 29: 280–329, Journal of Ecology 30: 147–201. [Google Scholar]
- 15. Roelofs JGM (1991) Inlet of alkaline river water into peaty lowlands: effects on water quality and Stratiotes aloides L. stands. Aquatic Botany 39: 267–293. [Google Scholar]
- 16. Caraco NF, Cole JJ, Likens GE (1993) Sulfate control of phosphorus availability in lakes. Hydrobiologia 253: 275–280. [Google Scholar]
- 17. Smolders AJP, Roelofs JGM (1995) Internal eutrophication, iron limitation and sulphide accumulation due to the inlet of river Rhine water in peaty shallow waters in The Netherlands. Archiv für Hydrobiologie 133: 349–365. [Google Scholar]
- 18. Hupfer M, Lewandowski J (2008) Oxygen Controls the Phosphorus Release from Lake Sediments –a Long-Lasting Paradigm in Limnology. International Review of Hydrobiology 93: 414–432. [Google Scholar]
- 19. Geurts JJM, Smolders AJP, Banach AM, van de Graaf JPM, Roelofs JGM, et al. (2010) The interaction between decomposition, N and P mineralization and their mobilisation to the surface water in fens. Water Research 44: 3487–3495. [DOI] [PubMed] [Google Scholar]
- 20. Smolders AJP, Lamers LPM, Moonen M, Zwaga K, Roelofs JGM (2001) Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry 54: 219–228. [Google Scholar]
- 21.Wetzel RG (2001) Limnology: Lake and River Ecosystems. Academic Press 3, An Imprint of Elsevier, USA: 1006 pag. [Google Scholar]
- 22. Beltman B, Rouwenhorst TG, Van Kerkhoven MB, Van Der Krift T, Verhoeven JTA (2000) Internal eutrophication in peat soils through competition between chloride and sulphate with phosphate for binding sites. Biogeochemistry 50: 183–194. [Google Scholar]
- 23. House WA (1999) The physio-chemical conditions for the precipitation of phosphate with calcium. Environmental Technology 20: 727–733. [Google Scholar]
- 24. Wu Y, Wen Y, Zhou J, Wu Y (2014) Phosphorus release from lake sediments: Effects of pH, temperature and dissolved oxygen. KSCE Journal of Civil Engineering 18 (1): 323–329. [Google Scholar]
- 25.Reigersman CJA (1946) Ontzilting van Noord-Holland. (Desalinisation of NorthHolland.) Rapport van de Commissie inzake het zoutgehalte der boezem- en polderwateren van Noord-Holland, ingesteld bij Besluit van den Minister van Waterstaat van 24 april 1939. Rijksuitgeverij, 's-Gravenhage: 191 pp.
- 26. Boxman AW, Peters RCJH, Roelofs JGM (2008) Long term changes in atmospheric N and S throughfall deposition and effects on soil solution chemistry in a Scots pine forest in the Netherlands. Environmental Pollution 156: 1252–1259. [DOI] [PubMed] [Google Scholar]
- 27. Van Gemerden H (1984) The sulphide affinity of phototrophic bacteria in relation to the location of elemental sulphur. Archiv für Mikrobiologie 139: 289–294. [Google Scholar]
- 28. Kamphake LJ, Hannah SA, Cohen JM (1967) Automated analysis for nitrate by hydrazine reduction. Water Research 1: 205–206. [Google Scholar]
- 29. Grasshoff K, Johannsen H (1972) A new sensitive and direct method for the automatic determination of ammonia in sea water. Journal du Conseil Permanent International pour l'Exploration de la Mer 34: 516–521. [Google Scholar]
- 30. Henriksen A (1965) An automated method for determining low-level concentrations of phosphate in fresh and saline waters. Analyst 90: 29–34. [Google Scholar]
- 31. O'Brien JE (1962) Automation in sanitary chemistry part 4: automatic analysis of chloride in sewage. Wastes Engineering 33: 670–682. [Google Scholar]
- 32. Golterman HL (1996) Fractionation of sediment phosphate with chelating compounds: a simplification, and comparison with other methods. Hydrobiologia 335: 87–95. [Google Scholar]
- 33. Boström B, Pettersson K (1982) Different patterns of phosphorus release from lake sediments in laboratory experiments. Hydrobiologia 92: 415–429. [Google Scholar]
- 34. Van Dijk G, Loeb R, Smolders AJP, Westendorp PJ (2013) Verbrakking in voormalig brak laag Nederland, bedreiging of kans? (Salinisation in former brackish Dutch lowlands, threat or opportunity?). H2O 46 (3): 1–5. [Google Scholar]
- 35. Dupraz C, Reid RP, Braissant O, Decho AW, Normanc RS, et al. (2009) Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews 96: 141–162. [Google Scholar]
- 36. Deinema MH, Habets LHA, Scholten J, Turkstra E, Webers HAAM (1980) The accumulation of polyphosphate in Acinetobacter spp. FEMS Microbiology Letters 9: 275–279. [Google Scholar]
- 37. Hupfer M, Uhlmann D (1991) Microbially mediated phosphorus exchange across the mud-water interface. Verhandlungen des Internationalen Verein Limnologie 24: 2999–3003. [Google Scholar]
- 38. Gunnars A, Blomqvist S, Johansson P, Andersson C (2002) Formation of Fe(III) oxyhydroxide colloids in freshwater and brackish seawater, with incorporation of phosphate and calcium. Geochimica et Cosmochimica Acta 66 (5): 745–758. [Google Scholar]
- 39. Blomqvist S, Gunnars A, Elmgren R (2004) Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: A matter of salt. Limnology and Oceanography 49 (6): 2236–2241. [Google Scholar]
- 40. Roden EE (2012) Microbial iron-redox cycling in subsurface environments. Biochemical Society Transactions 40: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 41. Imhoff A, Schneider A, Podgorsek L (1995) Correlation of viable cell counts, metabolic activity of sulphur-oxidizing bacteria and chemical parameters of marine sediments. Helgoländer Meeresunters 49: 223–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.