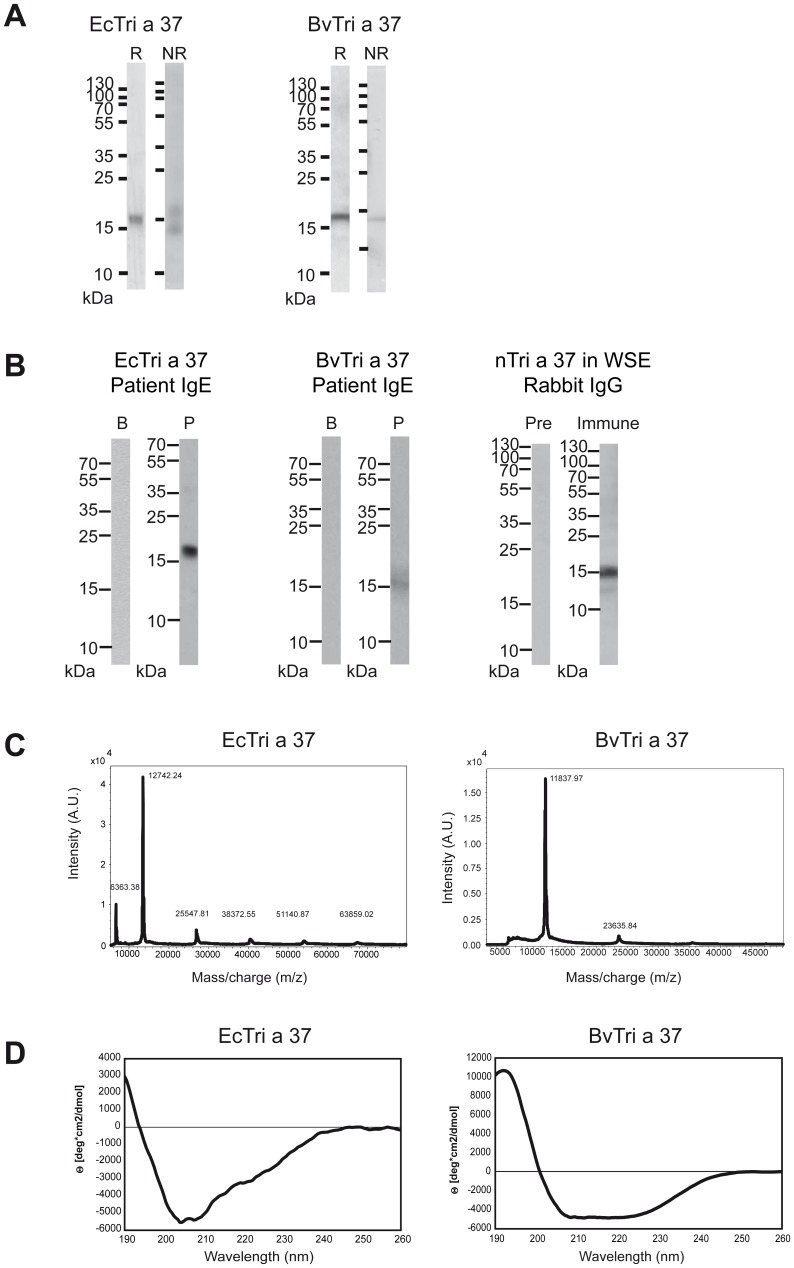
Figure 1. Characterization of purified Tri a 37 expressed in E.coli cells (EcTri a 37) and in baculovirus-infected insect cells (BvTri a 37) as well as detection of natural Tri a 37 in wheat seed extract.
(A) Coomassie brilliant blue-stained SDS-PAGE under reducing (lane R) and under non-reducing (lane NR) conditions. A protein molecular weight marker (kDa) is shown on the left side. (B) Detection of IgE-reactivitiy to Western-blotted EcTri a 37 and BvTri a 37, using serum from a wheat food allergic patient (P) and buffer (B) as a control. Western-blotted wheat seed extract was tested with Tri a 37-specific rabbit antibodies (Immune) or the corresponding pre-immune serum (Pre). Bound human IgE and rabbit IgG antibodies were detected with 125Iodine-labeled antibodies and visualized by autoradiography. Molecular weights are indicated in kilo Dalton (kDa). (C) Mass spectrometry of recombinant EcTri a 37 and BvTri a 37. The mass/charge ratios are shown on the x-axes, and the intensities are displayed on the y-axes as percentages of the most intensive signals obtained in the investigated mass range. (D) Circular dichroism analysis of recombinant EcTri a 37 and BvTri a 37. The spectra are expressed as mean residue ellipticities (θ) (y-axes) at given wavelengths (x-axes).