Abstract
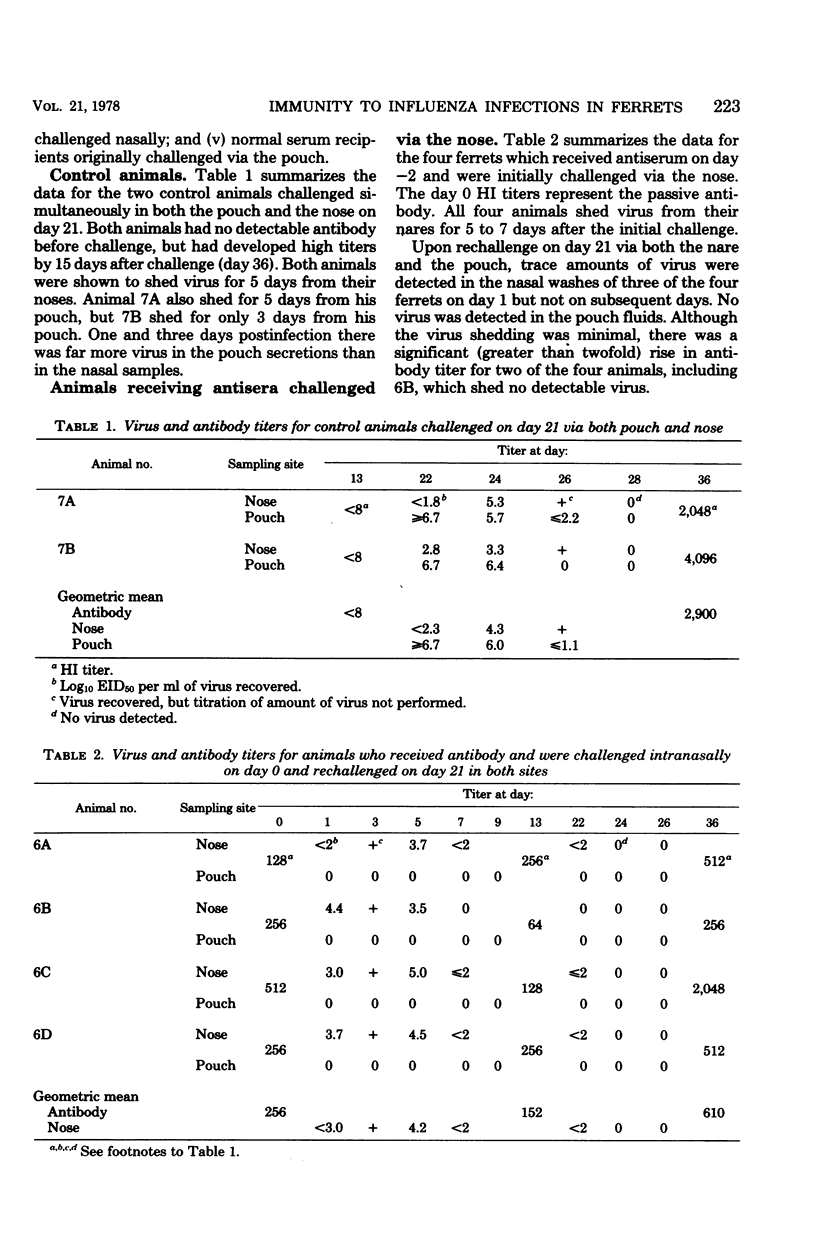
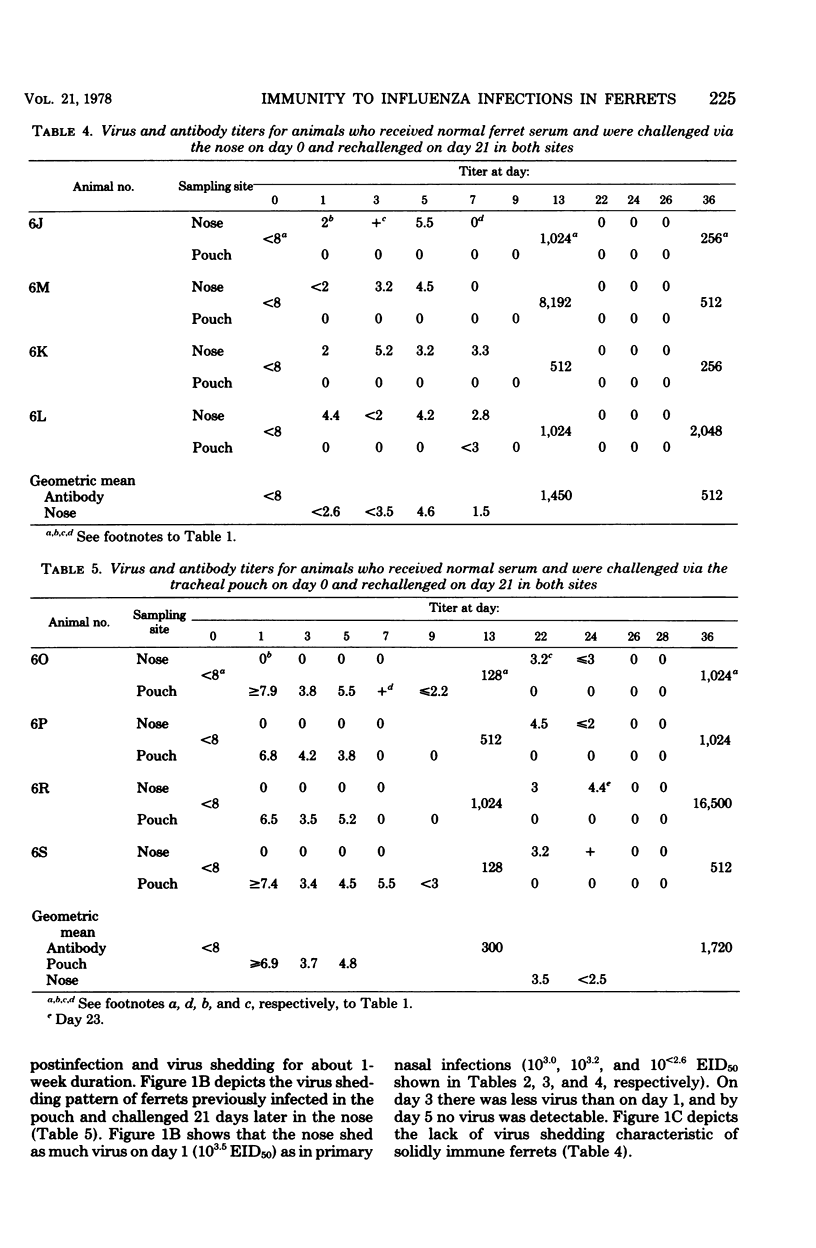
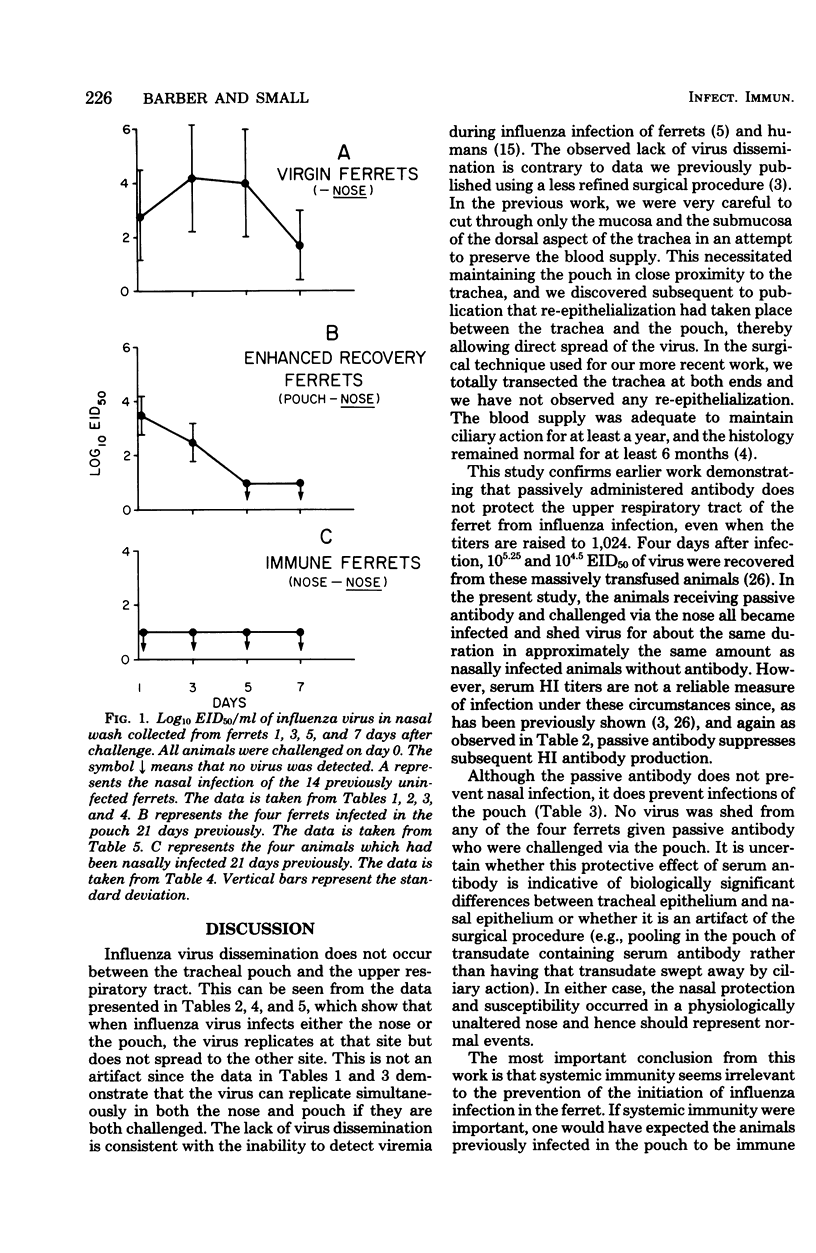
To establish whether immunity to influenza infection in the ferret is local or systemic, two sites of challenge were utilized: the nose and the anatomically isolated tracheal pouch. Infection of either site did not spread to the other site, and challenge of either site resulted in seroconversion by 13 days. Simultaneous challenge of both sites 21 days after the primary infection revealed that prior infection of the pouch prevented subsequent reinfection of the pouch, but not infection of the nose. Thus, systemic immunity did not prevent the initiation of nasal influenza infection in the ferret. However, the duration of virus shedding from the nose was reduced to half of that seen when ferrets were infected for the first time, showing that the prior pouch infection did lead to a more rapid recovery from the subsequent nasal infection. Passively administered anti-influenza antibody did not prevent or modify the nasal infection, but it did prevent the pouch infection. This is consistent with the observation that an initial infection of the nose prevented pouch infection upon challenge 21 days later. The prior nasal infection also prevented the subsequent nasal infection. These data suggest that immunity to acquisition of influenza infection in the ferret is a local phenomenon, whereas recovery from active infection is influenced by systemic immune mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Rossen R. D., Butler W. T., Kasel J. A. Neutralizing and hemagglutination-inhibiting activity of nasal secretions following experimental human infection with A2 influenza virus. J Immunol. 1967 Apr;98(4):724–731. [PubMed] [Google Scholar]
- Allan W. H., Madeley C. R., Kendal A. P. Studies with avian influenza A viruses: cross protection experiments in chickens. J Gen Virol. 1971 Aug;12(2):79–84. doi: 10.1099/0022-1317-12-2-79. [DOI] [PubMed] [Google Scholar]
- Barber H. W., Small P. A., Jr Construction of an improved tracheal pouch in the ferret. Am Rev Respir Dis. 1977 Jan;115(1):165–169. doi: 10.1164/arrd.1977.115.1.165. [DOI] [PubMed] [Google Scholar]
- Basarab O., Smith H. Quantitative studies on the tissue localization of influenza virus in ferrets after intranasal and intravenous or intracardial inoculation. Br J Exp Pathol. 1969 Dec;50(6):612–618. [PMC free article] [PubMed] [Google Scholar]
- Beare A. S., Tyrrell D. A., Hobson D., Howells C. H., Pereira M. S., Pollock T. M., Tyler L. E. Live influenza B vaccine in volunteers. A report to the Medical Research Council by their Committee on Influenza and Other Respiratory Virus Vaccines. J Hyg (Lond) 1969 Mar;67(1):1–11. doi: 10.1017/s002217240004136x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliano R. C., Small P. A., Jr Specific immunity to influenza virus in ferret organ cultures. Br J Exp Pathol. 1978 Feb;59(1):21–31. [PMC free article] [PubMed] [Google Scholar]
- Downie J. C., Stuart-Harris C. H. The production of neutralizing activity in serum and nasal secretion following immunization with influenza B virus. J Hyg (Lond) 1970 Jun;68(2):233–244. doi: 10.1017/s0022172400028709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson W. P., Jr, Rothenberg R., White P. W., Gwaltney J. M., Jr A comparison of subcutaneous, nasal, and combined influenza vaccination. II. Protection against natural challenge. Am J Epidemiol. 1971 Jun;93(6):480–486. doi: 10.1093/oxfordjournals.aje.a121282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadol N., Johnson J. E., 3rd, Waldman R. H. Respiratory tract cell-mediated immunity: comparison of primary and secondary response. Infect Immun. 1974 May;9(5):858–862. doi: 10.1128/iai.9.5.858-862.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki T., Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977 Jan;118(1):256–263. [PubMed] [Google Scholar]
- KAJI M., OSEASOHN R., JORDAN W. S., Jr, DINGLE J. H. Isolation of Asian virus from extrapulmonary tissues in fatal human influenza. Proc Soc Exp Biol Med. 1959 Feb;100(2):272–275. doi: 10.3181/00379727-100-24597. [DOI] [PubMed] [Google Scholar]
- LOOSLI C. G., HAMRE D., BERLIN B. S. Air-borne influenza virus A infections in immunized animals. Trans Assoc Am Physicians. 1953;66:222–230. [PubMed] [Google Scholar]
- Lien K. S., Jacobs J., Marcus E. A., Strik R. V. The protective effect of intranasal immunization with inactivated influenza virus vaccine. Postgrad Med J. 1973 Mar;49(569):175–179. [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Parish C. R. Regulation of the immune response by antibody. I. Suppression of antibody formation and concomitant enhancement of cell-mediated immunity by passive antibody. Cell Immunol. 1972 May;4(1):66–85. doi: 10.1016/0008-8749(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Morris J. A., Kasel J. A., Saglam M., Knight V., Loda F. A. Immunity to influenza to antibody levels. N Engl J Med. 1966 Mar 10;274(10):527–535. doi: 10.1056/NEJM196603102741001. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S., Shore S. L., McLaren C., Stuart-Harris C. Immunity to influenza in ferrets. I. Response to live and killed virus. Br J Exp Pathol. 1972 Apr;53(2):153–167. [PMC free article] [PubMed] [Google Scholar]
- Rossen R. D., Douglas G., Jr, Cate T. R., Couch R. B., Butler W. T. The sedimentation behavior of rhinovirus neutralizing activity in nasal secretion and serum following the rhinovirus common cold. J Immunol. 1966 Oct;97(4):532–538. [PubMed] [Google Scholar]
- Rott R., Becht H., Orlich M. The significance of influenza virus neuraminidase in immunity. J Gen Virol. 1974 Jan;22(1):35–41. doi: 10.1099/0022-1317-22-1-35. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Small P. A., Jr, Waldman R. H., Bruno J. C., Gifford G. E. Influenza infection in ferrets: role of serum antibody in protection and recovery. Infect Immun. 1976 Feb;13(2):417–424. doi: 10.1128/iai.13.2.417-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. B., Purcell R. H., Bellanti J. A., Chanock R. M. Protective effect of antibody to parainfluenza type 1 virus. N Engl J Med. 1966 Nov 24;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Mayner R. E., Barry D. W., Ennis F. A. Influenza virus infection in nude mice. J Infect Dis. 1976 Jan;133(1):91–94. doi: 10.1093/infdis/133.1.91. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Oya J., Ishida N. Effect of antilymphocyte serum on influenza virus infection in mice. Proc Soc Exp Biol Med. 1974 May;146(1):78–84. doi: 10.3181/00379727-146-38047. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L. Host defenses against influenza virus: the role of anti-hemagglutinin antibody. J Immunol. 1975 Aug;115(2):434–439. [PubMed] [Google Scholar]
- Waldman R. H., Coggins W. J. Influenza immunization: field trial on a university campus. J Infect Dis. 1972 Sep;126(3):242–248. doi: 10.1093/infdis/126.3.242. [DOI] [PubMed] [Google Scholar]
- Waldman R. H., Mann J. J., Small P. A., Jr Immunization against influenza. Prevention of illness in man by aerosolized inactivated vaccine. JAMA. 1969 Jan 20;207(3):520–524. doi: 10.1001/jama.207.3.520. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]


