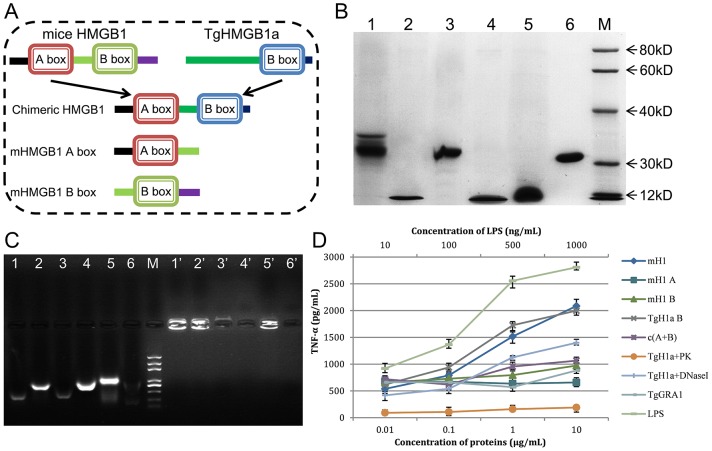
Figure 3. The TgHMGB1a box bound DNA fragments from E. coli and stimulated macrophages to release TNF-α.
A. To examine TgHMGB1a DNA binding ability and to compare that with mouse HMGB1, full length mouse HMGB1 (mH1), TgHMGB1a 4E (TgH1a B), independent mH1 A and B box domains and a chimeric mH1 A box-TgH1a B box (c (A+B)) were expressed in the prokaryote E. coli. B. Purified recombinant peptides were detected by SDS-PAGE. Lane 1, mH1; lane 2, TgH1a B; lane 3, chimeric c(A+B); lane 4, mH1 A; lane 5, mH1 A; and lane 6, TgGRA1 (control). M indicates the protein marker. C. Gel retardation experiments were carried out to identify bound DNA fragments. Lanes 1–6: denatured DNA-protein complexes from lanes 1–6 of Figure 3B . Lanes 1′–6′: natural complexes of lanes 1–6 of Figure 3B . M, DNA marker. All specific bands ranged from 200 to 1000 bp. D. Endotoxin removed peptides stimulates macrophages to release TNF-α at indicated concentrations. Ana1 macrophages cells were stimulated with HMG peptides at the indicated concentrations, and the culture medium was assayed for TNF-α at 24 h after stimulation. LPS and TgGRA1 were used as controls at the indicated concentrations. Each bar indicates the mean ± SD of three independent experiments. TNF-α secretion induced by TgHMGB1a is significant higher than controls (p<0.01).