ABSTRACT
Objectives
Nanothechnology found to be increasingly implemented in implantology sphere over the recent years and it shows encouraging effect in this field. The aim of present review is to compare, based on the recent evidence, the influence of various nanostructure surface modifications of titanium for implants, on osteoblasts proliferation.
Material and Methods
A literature review of English articles was conducted by using MEDLINE database restricted to 2009 - 2014 and constructed according PRISMA guidelines. Search terms included “Titanium implant”, “Titanium surface with nanostructure”, “Osteoblast”. Additional studies were identified in bibliographies. Only in vitro and/or in vivo studies on nano structured implant surfaces plus control sample, with specific evaluation method for osteoblasts proliferation and at least one Ti sample with nanostructure, were included in the review.
Results
32 studies with 122 groups of examined samples were selected for present review. Each study conducted in vitro experiment, two studies conducted additional in vivo experiments. All studies were dispensed by type of surface modification into two major groups; “Direct ablative titanium implant surface nano-modifications” with 19 studies and ”Nanocomposite additive implant surface modifications” with 13 studies. Overall 24 studies reporting on positive effect of nanostructured surface, 2 studies found no significant advantage and 6 studies reported on negative effect compared to other structure scales.
Conclusions
From examination of selected articles we can notice marked advantage in implementation of various nanostructures onto implant surface. Yet for discovering the ultimate implant surface nanostructure, further comparable investigations of Ti surface nanostructures need to be done.
Keywords: dental implants, nanotechnology, nanostructured materials, osteoblasts, cell proliferation
INTRODUCTION
Dental implant treatment is very widely spread and reliable treatment that provides good clinical results with high success rates over 90% [1-2].
Titanium is commonly used as an implant material as it has high biocompatibility and bonding ability with the bone. These characteristics were found in 1952 by the Swedish scientist Per-Ingvar Brånemark [3]. Since then, the results of many studies have demonstrated that titanium has high biocompatibility. Titanium has no adverse effect on the human body and bonds readily with the new bone, which penetrates into the titanium surface [4,5].
Implant survival rate and prognosis depends on quality of osseointegration as more direct bone-to-metal interface take place without interposition of non-bone tissue [6].
However, differences in bonding force between the implant body and bone occur depending on the differences in surface structures of the implant. Titanium surfaces play an important role in affecting osseointegration of dental implants. Many studies have concluded that certain characteristics of the implant surface play an important role in altering the quality of osseointegration [7-9]. It is commonly thought that the slightly roughened implant surface allows better osseointegration compared with the smooth implant surface [10,11]. Moreover nanostructured materials have shown increased cell attachment over microstructured or smooth surfaces [12,13].
An essential role of osseointegration processes is played by osteoblast progenitor stem cells during recruitment, adhesion, proliferation, differentiation, and mineralized matrix deposition during bone regeneration phases [14,15].
Nanoporous topography tend to help the proliferation processes, acting directly on the selective adhesion of osteoblastic cells on the surface, which can accelerate the healing process around implants [16,17]. Low osteoblasts cell number and proliferation have been closely associated with negative results when considering it to osseointegration [18,19].
Many studies were conducted to investigate various implant surface nanostructures and their influence on cell behaviour as proliferation, in contrast to other scopes of surface structures dimensions [20]. Many studies were conducted to compare different nanostructured morphologies as well. However, the optimal implant surface nanostructure covering for osteoblasts proliferation is yet to be established.
The aim of the present review is to compare, based on the recent available evidence, the influence of various nanostructure surface modifications of titanium for implants, on osteoblasts proliferation.
MATERIAL AND METHODS
Protocol and registration
The review is registered in international prospective register of systematic reviews ‘PROSPERO’ [21]. The protocol can be accessed at:
http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014009436
Registration number: CRD42014009436.
Eligibility criteria
Types of studies
The review included laboratory research studies, in vitro studies that using cells from human or animals and in vivo studies on animals. Studies published on English language between January 2009 and June 2014 with various evaluation methods for osteoblasts proliferation and various evaluation intervals between hours and days. Letters, reviews and abstracts were excluded.
Information sources
The information source was MEDLINE (PubMed) database.
Search
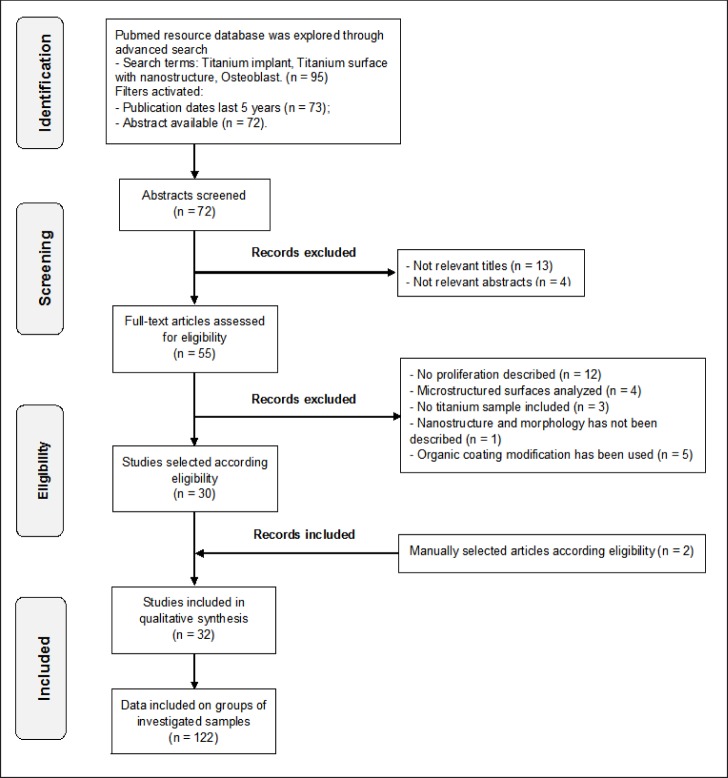
Pubmed resource database was explored through advanced search. The search terms those were used: “Titanium implant”, “Titanium surface with nanostructure”, “Osteoblast”. For more recent and updated information, search included only articles that were publicated from January 2009 to June 2014 to ensure sensitivity of the review. Additional simultaneous manual screening for related articles was performed. (Figure 1) illustrates the flow diagram of present articles selection according to PRISMA guidelines [22].
Figure 1.
Flow diagram of studies selection according PRISMA guidelines.
Study selection
Inclusion and exclusion criteria
Inclusion criteria for the selection were:
In vitro and/or in vivo studies.
Nano structured implant surface + control sample.
Studies with specific evaluation method for osteoblasts proliferation.
At least one Ti sample with nanostructure must be included in the study.
Exclusion criteria for the selection were:
No osteoblasts proliferation described.
Investigation of microstructured sufaces.
Nanostructure and morphology has not been described.
Organic coating has been used.
The search displayed 97 results from which 72 abstracts were screened (Figure 1). A total of 55 articles were reviewed in full. Preliminary exclusion was made by the title and its relevancy, later by abstract and its relevancy. Finally, articles that did not meet the inclusion and exclusion criteria, where filtered as followed: No proliferation described (n = 12), Microstructured surface analyzed (n = 4), No titanium sample included (n = 3), Nanostructure and morphology has not been described (n = 1), Organic coating has been used (n = 5).
Additional manual selection from references according eligibility was performed (n = 2).
Data collection process
Data was independently extracted from reports in form of variables according the aim and themes of present review as listed onwards.
Data items
Variables on which data were sought are as follow: ”TYPE OF STUDY”, indicates whether it was in vivo or in vitro or both and materials respectively. “SAMPLE”, describes the number of particular investigated samples in the study and its singularity (e.g., A-machined, B-polished, C-acid etched). “TOPOGRAPHY”, describes the nanoscale topography of the nanostructures on the surface of the sample, can be interrelated with SAMPLE description of nanostructure (e.g., nanograins 100nm). “EVALUATION”, describes evaluation methods and duration of osteoblasts proliferation cultured on the sample (e.g., Histology, 24, 48 and 72 hours).”RESULT”, describes the impact of surface structure on osteoblast proliferation.
Description of nanostructure peculiarities can vary from study to study and may be located under SAMPLE column or TOPOGRAPHY or both.
Risk of bias assessment
Risk of bias (e.g., lack of information or selective reports on variables of interest) was assessed on study level. The risks were indicated as lack of precise information of interest in each individual study that can blind the reader from particular information about examined samples.
The Cochrane Collaboration’s tool for assessing risk of bias [23] was used to assess bias across the studies that can affect cumulative evidence. Particularly “Blinding of outcome Assessment” and “Selective reporting”.
Synthesis of results
Relevant data of interest according stated previously variables, was collected and organized in two tables that divided according type of implant surface modification. The tables include results according individual evaluation of osteoblast proliferation.
Additional analyses
Separation of articles by their samples fabrication methods of nanostructured surfaces into two groups can provide possibility for simple comparison. Numbers of samples that provide positive or negative effect on osteoblasts proliferation are assessed in each article for each modification method. In addition samples of three topography types (e.g., control, microstructured and nanostrucruted) are included in each modification methods groups for better understanding of nanostructured surface superiority.
Quantitative and relative comparison between examined groups of samples and illustration of their relative efficiency in cells proliferation by means of diagrams.
RESULTS
Study selection
The search displayed 97 results from which 72 abstracts were screened. A total of 55 articles were reviewed in full. Preliminary exclusion during screening stage was made by the titles and abstracts relevancy (n = 13) and (n = 4) respectively. During eligibility stage articles that did not meet the inclusion and exclusion criteria, where filtered as followed: No proliferation described (n = 12), Microstructured surface analyzed (n = 4), No titanium sample included (n = 3), Nanostructure and morphology has not been described (n = 1), Organic coating has been used (n = 5). Additional relevant articles were added after manual selection from references according eligibility (n = 2). Finely 32 studies with 122 groups of examined samples were included in present review (Figure 1).
Study characteristics
All 32 studies finally selected for the review were in vivo and in vitro studies published in English with description of osteoblast proliferation. Each study conducted in vitro experiment by culturing osteoblasts on several investigated samples and controls when three studies conducted additional in vivo experiments. All authors except four, reported on conducting the experiments at least twice to ensure statistical validity.
The duration of osteoblasts proliferation evaluation varied from 2 hours to 14 days across all the studies except three articles of Gittens et al. [24-26] where evaluation was performed after culture confluence. The main evaluation methods for osteoblasts proliferation across the studies were: histology, MTT assay [27], alamarBlue™ assay [28], DNA assay and WST-1 or BrdU marker evaluations [29,30], additional visual evaluation by scanning electron microscope (SEM) was described by six authors, when Tetè et al. [31] uses SEM as main evaluation method.
All examined studies were assessed for specific variables described previously and were further divided into two groups characterized by type of implant surface modification. The division provided better understanding of nanosurface structure characteristics and contributed to sensitivity of the review.
First group “Direct ablative titanium implant surface nano-modifications” deals with titanium implant samples that were treated by various methods directly without addition of other materials to the implant surface, include 19 studies with 70 samples (Table 1).
Table 1.
Direct ablative titanium implant surface nano-modifications
|
Author
|
Structure (sample) |
Topography |
Type |
Evaluation |
Result |
|---|---|---|---|---|---|
|
Gittens et al. 2012 [24] |
PT-smooth SLA-sand blast acid etched nmPT-nano mod nmSLA-nano mod s.blast etch |
~5 nm - Not indicated ~20 nm - Not indicated (Only average roughness indicated from graph) |
In vitro MG63 Human osteoblast-like |
- DNA assay (cell amount)
Evaluated after confluence At least x 2 times |
Cell number, which decreases as cells transition from a proliferative to a more mature state, was lower for MG63s on the microrough surfaces compared to the microsmooth control, with the lowest levels on the combined microrough SLA and nanostructured nmSLA surfaces. P < 0.05 |
|
Gittens et al. 2012 [25] |
sTiAlV - microsmooth rTiAlV - microrough nmsTiAlV-nanomod nmrTiAlV-nanomod |
- some micro scale - some sub micro scale - 73 nm - 61 nm |
In vitro primery HOBs |
- Histology (Z1 Coulter particle counter) Evaluated after confluence At least x 2 times |
Osteoblast cell number, which decreases in differentiated cells due to a transcriptionally-restricted transition between proliferation and differentiation, was lower on the microrough surfaces, with the lowest levels on the combined microrough and nanostructured nmrTiAlV, biggest on sTiAlV. P < 0.05 |
|
Gittens et al. 2011 [26] (manually selected) |
- PT - SLA - nmPT (45 m) protuberance - nmPT (90 m) - nmPT (180 m) coarser str. - nmSLA |
- 0.43 µm roughness - 14 ± 6 nm roughness - 40 to 200 nm - 40 to 360 nm - 500 to 1000 nm - 18 ± 3 nm roughness |
In vitro MG63 cell |
- DNA assay Evaluated at confluence At least x 2 times |
The number of MG63 osteoblast from DNA measurements for the nmPT, SLA and nmSLA samples were lower than for the PT. This reduction in cells paralleled an increase in mean nanoscale roughness (nmPT vs. PT) and the microscale roughness (SLA and nmSLA vs. PT). P < 0.05
|
|
Tetè et al. 2011 [31] |
- Sandblasted - Blast+acidetched - Full contact coverage (galvanotactic anodizing process in a phosphate-sulfate) |
- 10 to 20 µm particles - Micro and nanopores 2 to 150 nm - Circular pores 10 to 700 nm (Mostly roughness explained) |
In vitro hDPaSCs OB like cells |
- Histology - SEM (prolif) 20 or 7 days |
After 7 days, culturing onto FCC titanium coating was possible to evaluate a higher number of cells growing on the titanium surface, distributed around more samples areas, and the typical net morphology tended to form a confluent layer. After 20 days, SEM analysis of FCC coating showed a great amount of cell proliferation. |
|
Zuo et al. 2013 [33] |
- machined Ti plates (Ti-m) - polished Ti plates (Ti-p) - DBD Ti plate (Ti-tr) |
- Ti-m less particles ~1 μm pores - Ti-p very sparse round nanoparticles ~1 μm pores - spherical nanoparticles 50 to 125 nm |
In vitro MC3T3-E1 Pre OB |
- Histology 24, 48, and 72 h - Hemocytometer x 3 times |
Initially dielectric barrier discharge (DBD) modification significantly enhanced cell adhesion, spread, and proliferation of preosteoblasts with no negative effects on cell differentiation. At 72 h, there was no remarkable difference between three groups. P < 0.05 and P < 0.01 |
|
Tsukimura et al 2011 [34] |
- Acid etched TI - Acid etched+TiO deposits - Acid etched+TiO deposits+UV treated |
- Microscale - 100 nm, 300 nm, 500 nm - 100 nm, 300 nm, 500 nm + UV |
In vitro Rat BMCs |
- Histology - WST-1 (density) - BrdU proliferation assay Day 3 |
Increase in the proliferative activity of cells on the nanonodular surfaces both before and after UV treatment, with that on the 300 nm nanonodules being the greatest. P < 0.05
|
|
Kubo et al. 2009 [35] |
- A Micropits - B micropits+nanonoduls - C micropits+nanonoduls - D micropits+nanonoduls |
- 0.5 to 1.5 μm - 100 nm - 300 nm - 500 nm |
In vitro Rat OB |
- WST-1 6, 24 h 2, 5 days - BrdU marker |
Cell density measured at culture days of 2 and 5 was substantially greater on the surfaces with nanonodules. The result of the BrdU incorporation per cell at day 2 confirmed the increased proliferation on the nanonodular surfaces, with the greatest one on the 300 nm nanonodules. P < 0.01 |
|
Han et al. 2011 [36] |
- Ti6Al4V Smooth - Ti6Al4V porous |
- Micro scale scratches - 10 to 20 nm grains |
In vitro neonatal rat calvaria OB |
- Histology - MTT assay 1, 3, 7, 10 and 14 days |
The growth curves showed that the osteoblasts on nanophase Ti6Al4V substrate appeared to have a not only higher but also longer growth phase for cell proliferation than those cultured on any of other surface. P < 0.01 |
|
Yu et al. 2010 [37] |
- Smooth-Ti - TN unannealed - Annealed 450 °C - Annealed 550 °C |
- Not indicated ~80 nm ~80 nm ~80 nm |
In vitro MC3T3-E1 Mouse pre OB |
- MTT assay 24, 48 and 72 h |
The proliferation of osteoblast cultured on anatase or anatase/rutile nanotube layersd showed significantly higher than smooth layer and amorphous nanotube layers, which means the crystal structure of nanotube layers can over-ride the chemistry effect and plays a main role in cell proliferation and mineralization. P < 0.05 |
|
Zhao et al. 2010 [38] |
- Smooth - acid-etched - etch/anod 5 V - etch/anod 20 V |
- Not indicated (smooth) - Micropits ~15 nm NT ~80 nm NT |
In vitro PRCOB |
- MTT assay 1, 4 and 7 days |
After 7 days cell number on the acid-etched/20 V anodized surface is observed to be slightly higher. Addition of nanotubes to microstructured surface enhances osteoblast behaviors with nearly all the cell functions retained or promoted. P < 0.05 |
|
Zhao et al. 2011 [40] |
Polished 5 V anodized 20 V anodized |
- Not indicated ~25 nm nanonet textur ~80 nm nanotubular texture |
In vitro PRCO (Primery rat calvarial ob) |
- Histology - SEM - MTT assay 30, 60, 120 m 1, 4, 7 days |
No significant difference in ad cell numbers on the 5 V, 20 V and polished is observed after 30, 60, 120 min and 1, 4, and 7 days except cell proliferation on the 5 V anodized surface is a little lower than on the other two Ti surfaces at days 1 and 4 P < 0.01 |
|
Xia et al. 2012 [41] |
- Nanotubes - Micropores - Flat (control) |
~100 nm ~ 10 to 20 μm - Shallow pits grooves |
In vitro MG63 cells In vivo (rabit) |
- Histology - MTT assay 1, 4, 7 days x times n = 9 |
More osteoblasts aggregated on the surface of TiO2 nanotube. This result was in accordance with the increased cellular proliferation on the TiO2 nanotubes observed in the In vitro study. Results showed increased proliferation on TiO2 nanotube than on microporous or polished Ti plates. P < 0.05 |
|
Brammer et al. 2009 [42] |
A-Ti B-TiO2 nanotubes B-TiO2 nanotubes C-TiO2 nanotubes D-TiO2 nanotubes |
- Not indicated - 30 nm - 50 nm - 70 nm - 100 nm |
In vitro MC3T3-E1 |
- Histology 2, 12, 24, 24 h 7 days - MTT 24, 48 h |
The number of adhered cells on the smallest 30 nm diameter nanotubes was notably higher than all the other sizes of nanotubes, but the cells started to be more elongated on nanotube diameters above 70 nm. P < 0.05 |
|
Zhang et al. 2012 [43] (manually selected) |
- Ti-control - Ti-6h small size sawtooth nanonetwork - Ti-24h large size sawtooth nanonetwork |
- Not indicated ~10 nm, 100 to 200 nm distance. ~30 nm, 200 to 300 nm distance. |
In vitro Rat BMMSCs |
- Histology(prolif) (laser scanning microscope) 1, 4, 7 days x 3 times |
The large nano-sawtooth structur approximately 30 nm produced the largest cell responses, including adhesion, proliferation, and differentiation properties. P < 0.05 and P < 0.01 |
|
Rani et al. 2012 [44] |
Nanotube (NT) Nanoscaffold (NS) Nanoleaf (NL) Nanoneedles (NN) polished Ti (contr) |
- Diameter 60 to 80 nm - roughness 166 nm - roughness 228 nm - roughness 940 nm - Not indicated |
In vitro pHOB In vivo (rats) |
- Histology - SEM - alamarBlueTM assay (prolif) 3, 5, 7 days |
On days 5 and 7, the proliferation rate was higher on the nanoleafy surface amongst all. These results correlate with the enhanced protein adsorption on nanoleafy samples. cells grown on NN surfaces showed a significant reduction in proliferation, despite high protein adsorption. P < 0.05 |
|
Zhuang et al. 2014 [45] |
- Smooth - SLA - micro, macro pits - SB-AH1 - nanoneedles - SB-AH2 - nanoporous |
- 0.4 ± 0.05 µm rough - 1.97 ± 0.19 µm rough - 0.94 ± 0.04 µm rough - 1.31 ± 0.06 µm rough (only roughness is indicated) |
In vitro MC3T3-E1 In vivo Rabits |
- CCK-8(WST8) 1, 3, 5, 7 days - Micro-CT 4 and 8 weeks |
Proliferation assay showed increased proliferation for SB-AH1 and SB-AH2 when SB-AH2 statistically better (P < 0.05). I Vivo study showed higher bone volume on SB-AH1 and SB-AH2 as well. |
|
Ross et al. 2013 [57] |
A polished B bead blasted C anod-sulfuric D anod+hydrofl anod E anod+hydrof etche |
- striations 1 to 2 μm - grain structure - rounded feature 2 μm - pore size of 1 to 2 μm - numerous nanometer features |
In vitro HOBs |
- Histology - Auto T4 Cellometer 1, 4, and 7 days x 3 times |
Anodization of Ti6Al4V using sulfuric acid followed by hydrofluoric acid with microporous surface 1 to 2 μm in diameter, and this promoted osteoblast densities D > E P < 0.01 |
|
Yu et al. 2014 [58] |
- Smooth - nano-foveolae |
- Not indicated - 10 to 20 μm grains with 80 nm nano-foveolae structures. |
In vitro MC3T3-E1 Mice pre OB |
- MTT 24, 72 h |
The proliferation rate of preosteoblasts was statistically similar at 24 h and statistically lower on nano-foveolae structures at 72 h. P < 0.05 |
|
Hori et al. 2010 [62] |
- Smooth (machined) - Micropits - TiO2 micro-nano-hybrid |
- Not indicated - 0.5 to 1.5 μm -198.5 ± 22.3 nm (nanonoduls) |
In vitro Rat OB |
- WST-1 6, 24 h 2, 5 days - BrdU marker |
The addition of nanonodules to the micropits, increased the number of cells two to three times to a level even greater than on the machined surface at 5th day. The result of the BrdU incorporation per cell at day 2 confirmed that proliferative activity of osteoblasts was impaired on the micropitted surface, whereas the proliferation on the micro-nano-hybrid surface was raised to a level equivalent to smooth surface. P < 0.05 |
Second group ”Nanocomposite additive implant surface modifications” deals with samples that were treated by addition of variable non organic particles, includes 13 studies with 50 samples, showed in (Table 2).
Table 2.
Nanocomposite additive implant surface modifications
|
Author
|
Structure (sample) |
Topography |
Type |
Evaluation |
Result |
|---|---|---|---|---|---|
|
Cecchinato et al. 2013 [32] |
- A-Not porous - B-Mesoporous - C-Mesoporous + Mg |
- Not indicated ~6 nm pore ~6 nm pore |
In vitro hFOB (human fetal OB) |
- MTT assay at 24 h - SEM 1 to 24 h x 3 times |
Three-dimensional nanostructure of TiO2 coatings as well as the wider specific surface area given by the presence of pores positively influence the osteoconductivity of titanium compared with the noncoated surfaces. P < 0.05 |
|
Zhao et al. 2011 [39] |
- TiO2-NT - NT-Ag 0.5M - NT-Ag 1.0 M - NT-Ag 1.5 M - NT-Ag 2.0 M - Flat-ctrl |
- NT-130 nm - NT-130 nm - NT-130 nm - NT-130 nm - NT-130 nm - Not indicated (All Ag particles 10 to 20 nm amount increase with concentration) |
In vitro Primary rat OB |
- Histology - DNA analysis 1 and 4 days |
After 4 days of culturing, the cell number on TiO2-NTs is smaller than that on flat Ti. Those on the NT-Ag samples are even smaller and the amounts correlate with the silver concentrations. NT-Ag structure shows some cytotoxicity, it can be reduced by controlling the Ag release rate. P < 0.01 |
|
Zhao et al. 2013 [46] |
- TiO2 coating - Nb2O5 doped TiO2 - SiO2 doped TiO2 coating |
- grains < 50 nm - nanoplates - hairy protrusions |
In vitro Primery HOBs |
- alamarBlue™ assay - SEM 2 to 24 h, 3 d, 7 d, 14 d |
TiO2 coating with Nb2O5 enhanced primary human osteoblast adhesion and promoted cell proliferation P < 0.05 |
|
Roy et al. 2011 [47] |
- HA coating Ti - Sr-HA coating Ti - Mg-HA coating Ti |
- 23 ± 3.9 nm grains - 21.6 ± 3.7 nm grain - 24.6 ± 5.3 nm grain |
In vitro hFOB1.19 cells |
- Histology - MTT assay 3, 7, 11 days x 3 times |
hFOB cell proliferation was accelerated on the Sr-HA coatings compared to pure HA or Mg doped HA coatings at all periods. P < 0.05 |
|
Zhou et al. 2013 [48] |
- Nanogranulated TiO - S67 interspace - S96 interspace - S137 interspace (Strontium-doped hydroxyapatite nanorods with different spacing and nanogranulate On TiO) |
- Not indicated - diam 71.4 nm - diam 68.9 nm - diam 67.6 nm |
In vitro hFOB1.19 (human fetal OB) |
- MTT assay 3, 7, 14 days |
Proliferation and differentiation of osteoblasts can be directly regulated by the interrod spacing of the Sr1-HA nanorods, which are significantly enhanced on the nanorod-shaped 3D patterns with interrod spacing smaller than 96 nm. P < 0.05 |
|
Bayram et al. 2012 [49] |
- Ti - An-Ti (nanotubes) - An-Ti-SBF (1 h) HA - An-Ti-SBF (2 h) HA - An-Ti-SBF (3 h) HA - An-Ti-SBF (5 h) HA - An-Ti-SBF (8 h) HA |
- Not indicated - 45 to 50 nm diameter 10 nm wall - spare 1 to 2 μm HA particles (The surface consisted of both apatite and titania nanotubes after 2 and 3hours of soaking) |
In vitro Saos-2/An1 (OB like human bone osteogenic sarcoma cell) |
- MTT assay 3, 5, 7 days x 3 times |
The percentage of cell viabilities cultured on the sample. Was greatest on An-Ti-SBF (3 h) with 45 to 50 nm diameter nanotubes and HA plaques, for all experimental days. P < 0.05 |
|
Portan et al. 2012 [50] |
- A-Ti - B-TiO2 nanotube - C-TiO2 nano+HA |
- Not indicated ~80 to 200 According SEM image. |
In vitro Human Bone marrow cells |
- Histology - SEM 1 week incubation |
There is an obvious positive change in the spreading of osteoblasts on HAp coated titania nanotubes layer comparing to cells on pure titanium or TiO2 nanotubes. |
|
Gu et al. 2012 [51] |
- A-bare Ti (contrl) - B-nanotub - C-nanotub+HA |
- Not indicated - 90 nm - 90 nm |
In vitro MC3T3-E1 |
- MTT proliferation assay 1, 4, 7 days |
The nanotubular surfaces showed significantly higher proliferation of preosteoblastic cells than the control after 7 days of culture. However, the proliferation rate was reduced on the HA-deposited nanotube surfaces during the incubation days compared with the untreated nanotubular and bare Ti. P < 0.05 |
|
Dimitrievska et al. 2011 [52] |
- UncoatTi64 (ctrl.) - HA coating - TiO2 coating - TiO2-HA coating |
- only roughness indicated - < 300 spherical crystallites - 20 to 30 nm Rod HA And 300 nm TiO spherical |
In vitro hMSC-derived OB |
- Histology - SEM - alamarBlue™ assay 2 to 6 h, 1 d, 7 d, 14 d |
Results revealed a higher metabolic activity and cell number of hMSC-ob on the TiO2-HA nanocomposite coatings when compared with the pure TiO2 and HA coatings, at 7 and 14 days of culture. P < 0.01 |
|
Wang et al. 2012 [53] |
- Ti controls - nHA coated Ti - B-SWCNT Ti - nHA+N-SWCNT Ti - nHA+B-SWCNT Ti - Glass references |
- Not indicated - 20 to 30 nm grains - 2 to 20 nm NT bundle - 1.52 nm diameter NT - 1.19 nm diameter NT |
In vitro hFOB |
- Histology (fluorescence microscopy) 1, 3 and 5 days x 3 times |
Significantly improved bone cell proliferation on the biomimetic nanocoatings after 3 and 5 day proliferation when compared to uncoated Ti and nHA coated Ti. nHA combined with B-SWCNTs or N-SWCNTs can achieve the highest osteoblast proliferation density. P < 0.05 |
|
Tran et al. 2010 [54] |
- Uncoated Ti - Low-nSe-Ti - Medium-nSe-Ti - High-nSe-Ti |
- low density - medium density - high density 80 nm selenium clusters |
In vitro PHCO primary human calvarial osteoblasts |
- Histology fluorescence microscopy 4, 17, 24, 40, 53 and 65 h x 3 times |
Healthy osteoblast densities significantly increased on High-nSe-Ti compared to uTi and Low-nSe-Ti. Cancerous osteoblasts, after three days,were much higher on uTi and Low-nSe-Ti than on High-nSe-Ti. P < 0.05 |
|
Mazzola et al. 2011 [55] |
- Uncoated - TiC-IPPA (IPPA - ion plating plasma assisted deposition) |
- Not indicated ~200 to 300 nm roughness |
In vitro hFOB 1.19 in vivo |
- DNA assay 24 h |
TiC covering Titanium substrate have beneficial effect on osteoblasts in vitro and in vivo . combination of morphology and chemistry in nanostructured TiC layer involves an increase of osteoblasts growth rate. |
|
Hu et al. 2013 [56] |
- A-Ti pure - B-TiO2- - C-TiO2/CaSiO3- |
- Not indicated - nano grains 20 to 100 nm - CaSiO3 nanocrystals |
In vitro MG63 |
- SEM 1, 3, 5 and 7 days - alamarBlue™ assay 1, 3, 5 and 7 days |
The proliferation rate and vitality of MG63 cells cultured on the TiO2/CaSiO3 coating are apparently higher than those on the TiO2 coating and pure Ti. P < 0.05, P < 0.01, P < 0.001 |
Risk of bias within studies
Only 22 from 32 studies fulfilled the expected markers of validity. The risk of bias that indicated within other 10 studies presented as lack of information values that grouped as followed: “Nano scale topography was not indicated”, “Evaluation methods with timing description” and “Significance of experiments indicated in the study result (P < 0.05) (Table 3).
Table 3.
Assessment of the risk of bias
|
Nano scale topography description |
Evaluation methods and timing description |
Significance of result indicated in the study (P < 0.05) |
|
|---|---|---|---|
|
Gittens et al. 2012 [24] |
No |
Not complete |
Yes |
|
Gittens et al. 2012 [25] |
Yes |
Not complete |
Yes |
|
Gittens et al. 2011 [26] |
Not complete |
Not complete |
Yes |
|
Tetè et al. 2010 [31] |
Not complete |
Yes |
No |
|
Rani et al. 2012 [44] |
Not complete |
Yes |
Yes |
|
Zhuang et al. 2014 [45] |
Not complete |
Yes |
Yes |
|
Portan et al. 2012 [50] |
No |
Yes |
No |
|
Dimitrievska et al. 2011 [52] |
Not complete |
Yes |
Yes |
|
Mazzola et al. 2011 [55] |
Not complete |
Not complete |
No |
|
Ross et al. 2013 [57] |
Not complete |
Yes |
Yes |
Synthesis of results
Present review focused on describing the studies, their results and qualitative synthesis rather than meta-analysis because the analyzed studies were not presented with clear quantitative results of osteoblasts proliferation, furthermore examined samples, evaluation methods and duration varied markedly.
Overall 24 studies with 89 examined samples from which 63 are various nanostractured patterns, reporting positive effect of 33 nanostrucure modified Ti samples, thus enhance osteoblasts proliferation on nanostructured features with significant differences (P < 0.05) between nanostructured surface, and microstructured or smooth control surfaces in each study.
No significant difference in positive effect on proliferation of cells cultured on nanostructured samples compared to microstructured or smooth control samples, was described in 2 studies with 6 examined samples; one study reports no significant differences between all three examined samples, another study result reveals equal proliferation on smooth sample and sample with micronanohybrid surface, whereas microstructured sample showed impaired proliferative activity.
Negative effect was described in 6 studies with 27 samples from which 15 are nanostructured samples, when results vary from, microstructured surface that promote osteoblasts proliferation to smooth or control samples showed better proliferation results.
Results by type of modification method, 19 belong to direct ablative titanium implant surface nano-modifications (Table 1) with 72 examined samples from which 41 are nanostructured and only 19 samples produce positive effect for cellular proliferation. 13 studies belong to nanocomposite additive implant surface modifications (Table 2), with 50 examined samples from which 40 are nanostructured with 25 samples reported as having significantly positive effect on osteoblasts proliferation.
Results of individual studies
Results of individual studies are shown in Table 1 and Table 2.
Risk of bias across studies
All studies examined didn’t show numerical data on the osteoblast proliferation results, what did not allow us to estimate precisely the advantage of one nanostructured sample on another.
All reviewed studies except two indicated significance of their results by (P < 0.05) what can be interpreted as study’s quality guaranty, but as mentioned previously, absence of quantitative results and calculations prevent conformation of significance and comparison across the studies by the reviewer.
In addition there are 10 studies with 41 examined samples were we could not find exact information as, nanoscale topography of the specific surface structure and/or evaluation timing. Review with and without inclusion of these studies found no differences in the patterns of our review results but only leave the reader blinded by lack of specific features of investigated sample. One study selectively reports on specific result from complete outcome [32].
Additional analysis
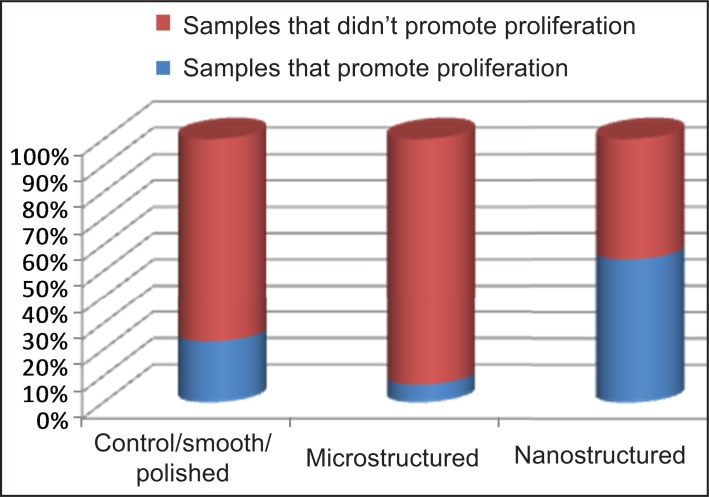
The division of articles into two groups by surface modification type contributed to better understanding of nanosurface structure characteristics and provide possibility for comparison between two main nanostructure modification methods (Table 4). Additionally the examined groups of samples can be compared for their relative influence on cell proliferation by surface topography. Percentage of groups of samples that promote proliferation in each surface topography, within nanostructures been the greatest illustrated in (Figure 2).
Table 4.
Division of samples by modification and topography
|
Articles division |
Direct ablative nanomodifications |
Nanocomposite additive modifications |
||||
|---|---|---|---|---|---|---|
|
Topography |
Control/smooth |
Microstructured |
Nanostructured |
Control/smooth |
Microstructured |
Nanostructured |
|
Sample amount |
16 |
15 |
41 |
10 |
0 |
40 |
|
Sample promote proliferation |
4 |
1 |
19 |
2 |
0 |
25 |
|
Percent |
25% |
6.7% |
46.3% |
20% |
- |
62% |
Figure 2.
Percentage of groups of samples that promote proliferation in each surface topography.
DISCUSSION
Summary of evidence
Overall, in most of reviewed articles 24 in number, nanostructured surfaces enhanced osteoblast proliferation compared to microstructured or smooth surfaces.
Dielectric barrier discharge (DBD) modification of Ti with spherical nanoparticles of 50 - 125 nm, significantly enhance cell proliferation, adhesion and spread without negative effect on differentiation [33].
Strong evidence delivered by two different studies that acid etched microfeature and TiO deposited samples with nanonodules of 100 nm, 300 nm and 500 nm increase in proliferation, with that on 300 nm being the greatest [34,35].
Porous Ti6Al4V substrate with 10 - 20 nm grains appeared to have a not only higher but also longer growth phase for cell proliferation [36].
Full contact coverage coating that was obtained by galvanotactic anodizing process in a phosphate-sulfate bath, (FCC) characterized by unique nanotopography of regular volcanoes with circular pores of 10 µm and 700 nm shows greater amount of cell proliferation than micro and nanopore of 2 µm and 150 nm [31].
Yu et al. [37] stated that crystal structure of nanotube layer can override the chemistry effect and plays a main role in cell proliferation, when anatase/rutile ~80 nm nanotube layers showed significantly higher proliferation than smooth layer and amorphous nanotube layers. This statement confirmed with evidence found in another study where ~80 nm nanotube surface increase cell number [38] when the same author reports on reduced cell number cultured on 130 nm nanotubes [39] and in other study of same author, no difference was found between ~80 nm
nanotubular pattern and polished sample [40]. Final conformation for nanotubes benefit in such dispute was found in in vivo and in vitro experiment [41].
One of the most advantageous nanotube diameters for better cell proliferation appears to be ~30 nm [42].
Additional nanofeature with ~30 nm was reported to have largest cell response, including proliferation adhesion and differentiation, as ~30 nm saw tooth nanonetwork surface with 200 - 300 nm inter tooth distance was examined [43].
Interesting findings were observed in additional combined in vivo and in vitro studies when one reports on higher cell proliferation rate on nanoleaf feature with roughness of 228 nm than on 60 - 80 nm nanotubs and significant reduction in proliferation on nanoneedle feature with 940 nm roughness [44], whereas another latest in vivo and in vitro study controversially reports on enhanced proliferation on nanoneedle structure [45].
Concerning nanocomposite materials incorporated into the implants surface we saw niobium (Nb2O5) doped TiO2 producing nanoplate structure that promote cell adhesion and proliferation [46]. Strong evidence brought to us in two studies that reported on strontium doped hydroxyapatite with 21.6 ± 3.7 nm grain morphology, accelerates cell proliferation [47,48]. Furthermore, cell proliferation can be directly regulated by Sr1-HA interrod spacing, with 71.4 nm interrod space three-dimensional patterns being the greatest.
Controversial results were noticed about incorporation of HA within nanotubes as two authors [49,50] claim that ‘there is an obvious positive change in the spreading and viability of osteoblasts on HA coated ~45 - 50 nm titania nanotubes layer comparing to cells on pure titanium or TiO2 nanotubes’ and in contrast Gu et al. [51] reports on reduced proliferation on 90 nm nanotubules with HA compared to same nanotubes without HA.
Another report reveals higher cell numbers on HA - TiO2 nanocomposite coated sample with 300 nm spherical TiO, 20 - 30 nm in diameter and 50 - 100 nm in length HA nanorods [52].
Significantly improved bone cell proliferation on the biomimetic nano coatings compared to uncoated Ti and nano-HA coated Ti was reported [53], as nano-HA combined with both magnetically and non magnetically treated ‘single walled carbon nanotubuls’ can achieve the highest osteoblasts proliferation density when diameter of SWCNT is 1.19 and 1.52 nm respectively.
Structures with 80 nm selenium clusters incorporated onto Ti implant surface, significantly increase healthy cell density compared to untreated Ti, on which cancerous osteoblasts found to be prevailed [54]. TiC layer deposited on Titanium sample by ion plating plasma assisted deposition, increase osteoblast growth rate as was claimed in an in vitro and in vivo studies [55]. Another nanocomposite material that succeeds to increase proliferation rate and vitality was TiO2/CaSiO3 which exists on the surface as CaSiO3 nanocristals on 20 - 10 nm TiO2 grains pattern [56].
Magnesium contained nanocomposite coatings found to be without any benefits for osteoblasts proliferation [30,47].
In contrast to 23 articles that describe positive effect of nanostructured surfaces, we are dealing with negative reports as follow. Ross et al. [57] reporting superiority of microstructure surface with pore size of 1 - 2 μm over nanostructured samples. Yu et al. [58] described lower proliferation rate on 80 nm nano-foveolae structure compared to smooth control sample. Gittens et al. [24-26] in three different studies describes negative results for micro and nano-modified samples compared to smooth and control surfaces in regard to osteoblasts proliferation, possibly due to transcriptionally-restricted transition. Transcriptionally-restricted transition between proliferation and differentiation is a process that forces osteoblasts to stop dividing once they start maturing [59-61]. Zhao et al. [39] from three articles included in this work, first reporting on significant smaller osteoblasts numbers cultured on nanotubules of 130 nm than on flat Ti sample, the number become even smaller when 10 - 20 nm Ag particles added to the surface. Second article finds no significant difference in cell numbers between polished sample, 25 nm nanonet texture and 80 nm nanotubular texture [40]. Third article reports on slightly enhanced cell number on the ~80 nm NT acid-etched/20 V anodized surface [38].
Hori et al. [62] describe relatively equivalent proliferation level on TiO2 smooth and 198.5 ± 22.3 nm TiO2 micronanohybrid.
After all, the most mentioned advantageous pattern is nanotubular structure with nanotube diameter of ~30 nm. Another superior morphological pattern across the studies was nanonoduls of 300 nm. Other nanostructures mentioned in our review need to be further investigated and compared for most advantageous nanoscale within each particular nanostructure. Furthermore this review analysed articles that present synergic effect of ablative and additive nanocomopsite surface coating to osteoblasts proliferation. Most articles that were including hydroxyapatite incorporation into Ti implant nanostructures describe obvious positive effect on cells proliferation especially when doped with strontium. Strontium doped HA nanorods appear to be beneficial with nanoscale of ~20 - 30 nm diameter and with interrod spacing of less than 96 nm. Beside strontium, nanostructures doped with niobium, selenium and CaSiO2 nanoparticles showed promising results, when selenium substrates suggesting a more favourable environment for healthy than cancerous osteoblasts. In contrast magnesium presence in the nanostructure poses some cytotoxicity.
Limitations
The main limitation of this overview is that the samples group types, the culture techniques and evaluation methods are not the same across studies and cannot be compared. All studies examined didn’t show numerical data on the osteoblast proliferation results, what did not allow us to estimate precisely the advantage of one nanostructured sample on another. All reviewed studies indicated significance of their results by (P < 0.05) what can be interpreted as study’s quality guaranty, but as mentioned previously, absence of quantitative results and calculations prevent conformation of significance and comparison across the studies by the reviewer. In addition there are 10 studies with 41 examined samples were we could not find exact information as, nanoscale topography of the specific surface structure and/or evaluation timing. Review with and without inclusion of these studies found no differences in the patterns of
our review results but only leave the reader blinded by lack of specific features of investigated sample.
CONCLUSIONS
From examination of selected articles we can notice marked advantage in implementation of various nanostructures onto implant surface. In our review 24 articles reporting on positive effect of nanostructured surfaces on osteoblasts proliferation, when 33 samples with particular nanostructures markedly enhance cell proliferation. Most of the examined nanostructures showed obvious positive impact on osteoblasts proliferation compared to other topography scales. Yet for discovering the ultimate implant surface nanostructure, further investigations of Ti nanopatterns with various nanoscales need to be done, moreover for reaching the most sensitive outcome, the experiments should be statistically compared, what can be achieved only when different studies will use the same concerted evaluation method for osteoblasts proliferation.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Lekholm U, Gröndahl K, Jemt T. Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin Implant Dent Relat Res. 2006;8(4):178-86. [DOI] [PubMed]
- 2.Jemt T. Single implants in the anterior maxilla after 15 years of follow-up: comparison with central implants in the edentulous maxilla. Int J Prosthodont. 2008 Sep-Oct;21(5):400-8. [PubMed]
- 3.Brånemark PI, Adell R, Breine U, Hansson BO, Lindström J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3(2):81-100. [DOI] [PubMed]
- 4.Satomi K, Akagawa Y, Nikai H, Tsuru H. Bone-implant interface structures after nontapping and tapping insertion of screw-type titanium alloy endosseous implants. J Prosthet Dent. 1988 Mar;59(3):339-42. [DOI] [PubMed]
- 5.Schmidt C, Kaspar D, Sarkar MR, Claes LE, Ignatius AA. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. J Biomed Mater Res. 2002;63(3):252-61. [DOI] [PubMed]
- 6.Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983 Sep;50(3):399-410. Review. [DOI] [PubMed]
- 7.Cooper LF. Biologic determinants of bone formation for osseointegration: clues for future clinical improvements. J Prosthet Dent. 1998 Oct;80(4):439-49. Review. [DOI] [PubMed]
- 8.Nanci A, Wuest JD, Peru L, Brunet P, Sharma V, Zalzal S, McKee MD. Chemical modification of titanium surfaces for covalent attachment of biologicalmolecules. J Biomed Mater Res. 1998 May;40(2):324-35. [DOI] [PubMed]
- 9.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999 Jun;13:38-48. Review. [DOI] [PubMed]
- 10.Fan Z, Jia S, Su JS. [Influence of surface roughness of titanium implant on core binding factor alpha 1 subunit of osteoblasts]. Zhonghua Kou Qiang Yi Xue Za Zhi. 2010 Aug;45(8):466-70. Chinese. [PubMed]
- 11.Jäger M, Zilkens C, Zanger K, Krauspe R. Significance of nano- and microtopography for cell-surface interactions in orthopaedic implants. J Biomed Biotechnol. 2007;2007(8):69036. [DOI] [PMC free article] [PubMed]
- 12.Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001 Jun;7(3):291-301. [DOI] [PubMed]
- 13.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004 Aug;25(19):4731-9. [DOI] [PubMed]
- 14.Piattelli A, Piattelli M, Romasco N, Trisi P. Histochemical and laser scanning microscopy characterization of the hydroxyapatite-bone interface: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1994 Mar-Apr;9(2):163-8. [PubMed]
- 15.Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. Biology of implantosseointegration. J Musculoskelet Neuronal Interact. 2009 Apr-Jun;9(2):61-71. Review. [PubMed]
- 16.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007 Jul;23(7):844-54. Epub 2006 Aug 14. Review. [DOI] [PubMed]
- 17.de Oliveira PT, Nanci A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials. 2004 Feb;25(3):403-13. [DOI] [PubMed]
- 18.Aita H, Hori N, Takeuchi M, Suzuki T, Yamada M, Anpo M, Ogawa T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009 Feb;30(6):1015-25. Epub 2008 Nov 29. [DOI] [PubMed]
- 19.Saito T, Hayashi H, Kameyama T, Hishida M, Nagai K, Teraoka K, Kato K. Suppressed proliferation of mouse osteoblast-like cells by a rough-surfaced substrate leads to low differentiation and mineralization. Mater Sci Eng C Mater Biol Appl. 2010 Jan;30(1):1–7. [DOI]
- 20.Palin E, Liu HN, Webster TJ. Mimicking the nanofeatures of bone increases bone-forming cell adhesion and proliferation. Nanotechnology. 2005;16(9):1828-35. [DOI]
- 21.Chien PF, Khan KS, Siassakos D. Registration of systematic reviews: PROSPERO. BJOG. 2012 Jul;119(8):903-5. [DOI] [PubMed]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg.2010;8(5):336-41. Epub 2010 Feb 18. Erratum in: Int J Surg. 2010;8(8):658. [DOI] [PubMed]
- 23.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. URL: http://www.cochrane.org/cochrane-interventions-handbook.
- 24.Gittens RA, Olivares-Navarrete R, Cheng A, Anderson DM, McLachlan T, Stephan I, Geis-Gerstorfer J, Sandhage KH, Fedorov AG, Rupp F, Boyan BD, Tannenbaum R, Schwartz Z. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013 Apr;9(4):6268-77. Epub 2012 Dec 8. [DOI] [PMC free article] [PubMed]
- 25.Gittens RA, Olivares-Navarrete R, McLachlan T, Cai Y, Hyzy SL, Schneider JM, Schwartz Z, Sandhage KH, Boyan BD. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials. 2012 Dec;33(35):8986-94. doi: 10.1016/j.biomaterials.2012.08.059. Epub 2012 Sep 16. [DOI] [PMC free article] [PubMed]
- 26.Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, Schwartz Z, Sandhage KH, Boyan BD. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011 May;32(13):3395-403. [DOI] [PMC free article] [PubMed]
- 27.Ciapetti G, Cenni E, Pratelli L, Pizzoferrato A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials. 1993 Apr;14(5):359-64. [DOI] [PubMed]
- 28.Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997 May 26;204(2):205-8. [DOI] [PubMed]
- 29.Li X, Darzynkiewicz Z. Labelling DNA strand breaks with BrdUTP. Detection of apoptosis and cell proliferation. Cell Prolif. 1995 Nov;28(11):571-9. [DOI] [PubMed]
- 30.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271-7. [DOI] [PubMed]
- 31.Han P, Ji WP, Zhao CL, Zhang XN, Jiang Y. Improved osteoblast proliferation, differentiation and mineralization on nanophase Ti6Al4V. Chin Med J (Engl). 2011 Jan;124(2):273-9. [PubMed]
- 32.Bayram C, Demirbilek M, Calişkan N, Demirbilek ME, Denkbaş EB. Osteoblast activity on anodized titania nanotubes: effect of simulated body fluid soaking time. J Biomed Nanotechnol. 2012 Jun;8(3):482-90. [DOI] [PubMed]
- 33.Tetè S, Mastrangelo F, Quaresima R, Vinci R, Sammartino G, Stuppia L, Gherlone E. Influence of novel nano-titanium implant surface on human osteoblast behavior and growth. Implant Dent. 2010 Dec;19(6):520-31. [DOI] [PubMed]
- 34.Cecchinato F, Xue Y, Karlsson J, He W, Wennerberg A, Mustafa K, Andersson M, Jimbo R. In vitro evaluation of human fetal osteoblast response to magnesium loaded mesoporous TiO(2) coating. J Biomed Mater Res A. 2013 Dec 12. [Epub ahead of print] [DOI] [PubMed]
- 35.Zuo J, Huang X, Zhong X, Zhu B, Sun Q, Jin C, Quan H, Tang Z, Chen W. A comparative study of the influence of three pure titanium plates with differentmicro- and nanotopographic surfaces on preosteoblast behaviors. J Biomed Mater Res A. 2013 Nov;101(11):3278-84. Epub 2013 Apr 29. [DOI] [PubMed]
- 36.Tsukimura N, Yamada M, Iwasa F, Minamikawa H, Att W, Ueno T, Saruwatari L, Aita H, Chiou WA, Ogawa T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials. 2011 Jul;32(19):4358-68. Epub 2011 Mar 21. [DOI] [PubMed]
- 37.Kubo K, Tsukimura N, Iwasa F, Ueno T, Saruwatari L, Aita H, Chiou WA, Ogawa T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscalehierarchy model. Biomaterials. 2009 Oct;30(29):5319-29. Epub 2009 Jul 9. [DOI] [PubMed]
- 38.Yu WQ, Zhang YL, Jiang XQ, Zhang FQ. In vitro behavior of MC3T3-E1 preosteoblast with different annealing temperature titania nanotubes. Oral Dis. 2010 Oct;16(7):624-30. [DOI] [PubMed]
- 39.Zhao L, Mei S, Chu PK, Zhang Y, Wu Z. The influence of hierarchical hybrid micro/nano-textured titanium surface with titania nanotubes on osteoblast functions. Biomaterials. 2010 Jul;31(19):5072-82. [DOI] [PubMed]
- 40.Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, Zhang Y, Wu Z, Chu PK. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011 Aug;32(24):5706-16. [DOI] [PubMed]
- 41.Zhao L, Mei S, Wang W, Chu PK, Zhang Y, Wu Z. Suppressed primary osteoblast functions on nanoporous titania surface. J Biomed Mater Res A. 2011 Jan;96(1):100-7. Epub 2010 Nov 4. [DOI] [PubMed]
- 42.Xia L, Feng B, Wang P, Ding S, Liu Z, Zhou J, Yu R. In vitro and in vivo studies of surface-structured implants for bone formation. Int J Nanomedicine. 2012;7:4873-81. Epub 2012 Sep 11. [DOI] [PMC free article] [PubMed]
- 43.Brammer KS, Oh S, Cobb CJ, Bjursten LM, van der Heyde H, Jin S. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009 Oct;5(8):3215-23. Epub 2009 May 15. [DOI] [PubMed]
- 44.Zhang W, Li Z, Liu Y, Ye D, Li J, Xu L, Wei B, Zhang X, Liu X, Jiang X. Biofunctionalization of a titanium surface with a nano-sawtooth structure regulates the behavior of rat bone marrow mesenchymal stem cells. Int J Nanomedicine. 2012;7:4459-72. Epub 2012 Aug 13. [DOI] [PMC free article] [PubMed]
- 45.Rani VV, Vinoth-Kumar L, Anitha VC, Manzoor K, Deepthy M, Shantikumar VN. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012 May;8(5):1976-89. Epub 2012 Jan 28. [DOI] [PubMed]
- 46.Zhuang XM, Zhou B, Ouyang JL, Sun HP, Wu YL, Liu Q, Deng FL. Enhanced MC3T3-E1 preosteoblast response and bone formation on the addition of nano-needle and nano-porous features to microtopographical titanium surfaces. Biomed Mater. 2014 Aug;9(4):045001. Epub 2014 Jun 19. [DOI] [PubMed]
- 47.Zhao X, Wang G, Zheng H, Lu Z, Zhong X, Cheng X, Zreiqat H. Delicate refinement of surface nanotopography by adjusting TiO2 coating chemical composition for enhanced interfacial biocompatibility. ACS Appl Mater Interfaces. 2013 Aug 28;5(16):8203-9. Epub 2013 Aug 19. [DOI] [PubMed]
- 48.Roy M, Bandyopadhyay A, Bose S. Induction plasma sprayed Sr and Mg doped nano hydroxyapatite coatings on Ti for bone implant. J Biomed Mater Res B Appl Biomater. 2011 Nov;99(2):258-65. Epub 2011 Jun 28. [DOI] [PubMed]
- 49.Zhou J, Li B, Lu S, Zhang L, Han Y. Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings. ACS Appl Mater Interfaces. 2013 Jun 12;5(11):5358-65. Epub 2013 May 21. [DOI] [PubMed]
- 50.Portan DV, Kroustalli AA, Deligianni DD, Papanicolaou GC. On the biocompatibility between TiO2 nanotubes layer and human osteoblasts. J Biomed Mater Res A. 2012 Oct;100(10):2546-53. Epub 2012 Apr 24. [DOI] [PubMed]
- 51.Gu YX, Du J, Zhao JM, Si MS, Mo JJ, Lai HC. Characterization andpreosteoblastic behavior of hydroxyapatite-deposited nanotube surface of titanium prepared by anodization coupled with alternative immersion method. J Biomed Mater Res B Appl Biomater. 2012 Nov;100(8):2122-30. Epub 2012 Jul 30. [DOI] [PubMed]
- 52.Dimitrievska S, Bureau MN, Antoniou J, Mwale F, Petit A, Lima RS, Marple BR. Titania-hydroxyapatite nanocomposite coatings support human mesenchymal stem cells osteogenic differentiation. J Biomed Mater Res A. 2011 Sep 15;98(4):576-88. Epub 2011 Jun 23. [DOI] [PubMed]
- 53.Wang M, Castro NJ, Li J, Keidar M, Zhang LG. Greater osteoblast and mesenchymal stem cell adhesion and proliferation on titanium with hydrothermally treated nanocrystalline hydroxyapatite/magnetically treated carbon nanotubes. J Nanosci Nanotechnol. 2012 Oct;12(10):7692-702. [DOI] [PubMed]
- 54.Tran PA, Sarin L, Hurt RH, Webster TJ. Differential effects of nanoselenium doping on healthy and cancerous osteoblasts in coculture on titanium. Int J Nanomedicine. 2010 May 13;5:351-8. [DOI] [PMC free article] [PubMed]
- 55.Mazzola L, Bemporad E, Misiano C, Pepe F, Santini P, Scandurra R. Surface analysis and osteoblasts response of a titanium oxi-carbide film deposited on titanium by ion plating plasma assisted (IPPA). J Nanosci Nanotechnol. 2011 Oct;11(10):8754-62. [DOI] [PubMed]
- 56.Hu H, Qiao Y, Meng F, Liu X, Ding C. Enhanced apatite-forming ability and cytocompatibility of porous and nanostructured TiO2/CaSiO3 coating on titanium. Colloids Surf B Biointerfaces. 2013 Jan 1;101:83-90. Epub 2012 Jun 29. [DOI] [PubMed]
- 57.Ross AP, Webster TJ. Anodizing color coded anodized Ti6Al4V medical devices for increasing bone cell functions. Int J Nanomedicine. 2013;8:109-17. Epub 2013 Jan 4. [DOI] [PMC free article] [PubMed]
- 58.Yu WQ, Xu L, Zhang FQ. The effect of Ti anodized nano-foveolae structure on preosteoblast growth and osteogenic gene expression. J Nanosci Nanotechnol. 2014 Jun;14(6):4387-93. [DOI] [PubMed]
- 59.Lian JB, Stein GS, Bortell R, Owen TA. Phenotype suppression: a postulated molecular mechanism for mediating the relationship of proliferation and differentiation by Fos/Jun interactions at AP-1 sites in steroid responsive promoter elements of tissue-specific genes. J Cell Biochem. 1991 Jan;45(1):9-14. Review. [DOI] [PubMed]
- 60.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993 Aug;14(4):424-42. Review. [DOI] [PubMed]
- 61.Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996 Apr;76(2):593-629. Review. [DOI] [PubMed]
- 62.Hori N, Iwasa F, Ueno T, Takeuchi K, Tsukimura N, Yamada M, Hattori M, Yamamoto A, Ogawa T. Selective cell affinity of biomimetic micro-nano-hybrid structured TiO2 overcomes the biological dilemma of osteoblasts. Dent Mater. 2010 Apr;26(4):275-87. Epub 2009 Dec 16. [DOI] [PubMed]