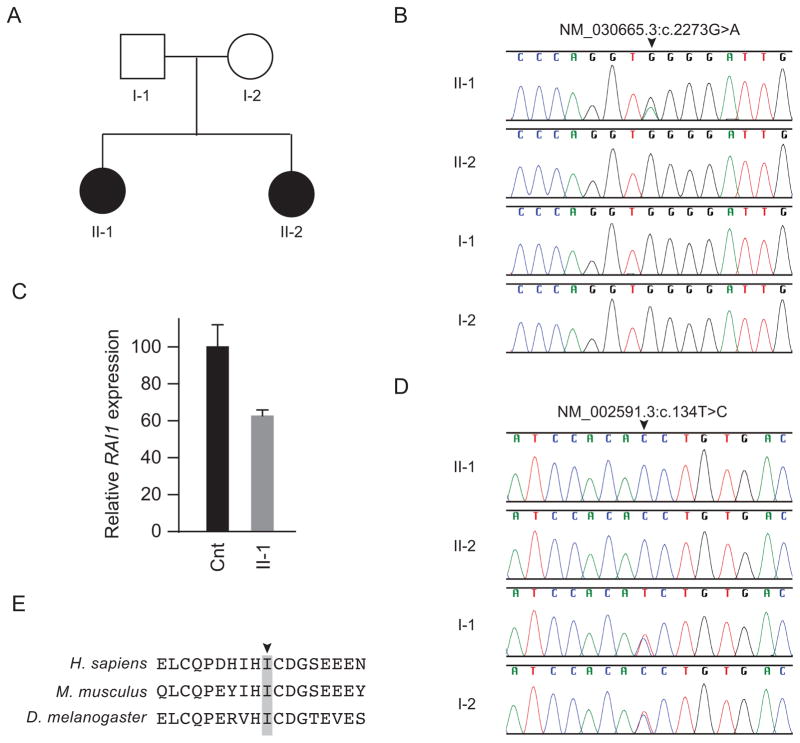
Fig. 2.
Identification and characterization of RAI1 and PCK1 mutations. (A) Family pedigree. (B) Sanger sequence traces showing a heterozygous RAI1 mutation (NM_030665:c.2273G>A, NP_109590.3:p.W758X) only in patient 1 (II-1). (C) Quantification of RAI1 steady state mRNA levels in cultured skin fibroblasts of patient 1 and an unaffected control. The nearly 50% decrease in RAI1 steady state mRNA levels in patient lymphoblastoid cells compared to unaffected control lymphoblastoid cells suggests that the p.W758X causes nonsense mediated mRNA decay. β-actin mRNA levels were used for normalization. (D) Sanger sequence traces showing that both patients (II-1 and II-2) had a homozygous PCK1 mutation (NM_002591.3:c.134T>C, NP_002582.3:p.I45T), whereas the parents (I-1 and I-2) were heterozygous for the mutation. (E) NP_002582.3:p.I45T alters an isoleucine (arrowhead) conserved across species to Drosophila melanogaster (see also Figure S-1 for more extensive species comparison, indicating this hydrophobic residue is conserved in the GTP utilizing enzymes all the way through Ascaris suum).