Summary
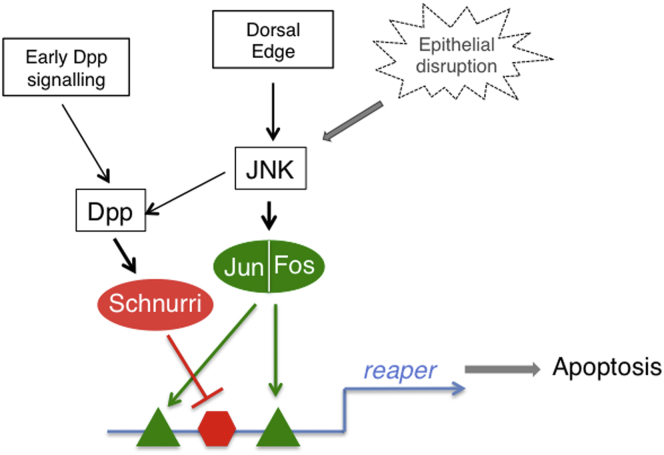
Jun N-terminal kinase (JNK) often mediates apoptosis in response to cellular stress. However, during normal development, JNK signaling controls a variety of live cell behaviors, such as during dorsal closure in Drosophila embryos. During this process, the latent proapoptotic activity of JNK becomes apparent following Dpp signaling suppression, which leads to JNK-dependent transcriptional activation of the proapoptotic gene reaper. Dpp signaling also protects cells from JNK-dependent apoptosis caused by epithelial disruption. We find that repression of reaper transcription by Dpp is mediated by Schnurri. Moreover, reporter gene analysis shows that a transcriptional regulatory module comprising AP-1 and Schnurri binding sites located upstream of reaper integrate the activities of JNK and Dpp. This arrangement allows JNK to control a migratory behavior without triggering apoptosis. Dpp plays a dual role during dorsal closure. It cooperates with JNK in stimulating cell migration and also prevents JNK from inducing apoptosis.
Graphical Abstract
Highlights
-
•
Dpp/BMP signaling prevents JNK from activating expression of proapoptotic reaper
-
•
The transcriptional repressor Schnurri mediates the antiapoptotic effect of Dpp
-
•
AP-1 and Schnurri binding sites integrate the inputs of JNK and Dpp on reaper
-
•
Dpp signaling ensures the survival of dorsal edge cells during dorsal closure
JNK often mediates apoptosis in response to cellular stress, yet JNK signaling controls a variety of live cell behaviors during normal development. In Drosophila embryos, JNK can direct dorsal closure because concurrent Dpp signaling, mediated by the repressor Schnurri, prevents activation of the proapoptotic gene reaper by JNK.
Introduction
Signaling by c-Jun N-terminal kinase (JNK) mediates one of the major stress response pathways (Chen, 2012, Stronach and Perrimon, 1999). Indeed, activation of JNK signaling often boosts or triggers apoptosis (Dhanasekaran and Reddy, 2008, Igaki, 2009, Leppä and Bohmann, 1999). JNK can exert its proapoptotic effect through phosphorylation of Jun, a component of the AP-1 transcriptional activator, or of other cellular proteins (Bogoyevitch and Kobe, 2006). It is important to note, however, that JNK signaling does not always trigger apoptosis (Weston and Davis, 2007) and has been shown to control nonapoptotic processes such as cytoskeletal rearrangements (Homsy et al., 2006), cell migration (Ríos-Barrera and Riesgo-Escovar, 2013), and cell proliferation (Shaulian and Karin, 2002) during development and regeneration. It is generally thought that the cellular context or the activity of other signaling pathways determines whether JNK signaling leads to apoptosis or not. In well-documented instances, this involves downregulation or blunting of JNK signaling itself, e.g., through the activity of Gadd45β, an NF-κB-induced factor (De Smaele et al., 2001, Papa et al., 2004), or by Puckered, a feedback inhibitor of JNK signaling (McEwen and Peifer, 2005). Mechanisms that dampen JNK’s proapoptotic influence without affecting core pathway activity have also been documented. For example, in the developing Drosophila eye, mitogen-activated protein kinase phosphorylates and destabilizes Hid, a proapoptotic protein transcriptionally activated by JNK signaling in this tissue (Bergmann et al., 1998). Another documented process involves transcriptional repression of hid, which would otherwise be overactivated by JNK in response to irradiation damage (Luo et al., 2007). Non-cell-autonomous protective mechanisms could also be at work. For example, the transcriptional modulator Schnurri limits radiation-induced tissue damage by recruiting macrophages through activation of the PDGF-related growth factor Pvf1 (Kelsey et al., 2012). All the aforementioned mechanisms have been shown to operate in response to cellular stress. However, so far, little is known about the regulatory processes that prevent JNK from causing apoptosis during normal development. One well-characterized nonapoptotic JNK-dependent developmental process is the morphogenetic movement of dorsal closure (Glise and Noselli, 1997, Hou et al., 1997, Riesgo-Escovar and Hafen, 1997). Here, we set out to investigate the molecular mechanisms that prevent JNK from activating apoptosis during this process.
Dorsal closure is a morphogenetic movement that closes a large gap left on the embryo’s dorsal side after germ band retraction. It involves the concerted movement of the dorsal epidermis toward the midline and requires both JNK and Dpp signaling. One current view is that JNK signaling at the leading edge promotes expression of Decapentaplegic (Dpp, a transforming growth factor β [TGF-β] homolog), which, in turn, orchestrates the cell shape changes required for dorsal closure (Fernández et al., 2007, Ríos-Barrera and Riesgo-Escovar, 2013). It is likely that a protective mechanism is at work at the leading edge since JNK does not trigger apoptosis there. Additional evidence that the dorsal epidermis is protected from apoptosis came from the analysis of crumbs (crb) (abbreviated as crb in genotypic descriptions) mutants, where JNK target genes are upregulated in response to loss of apicobasal polarity (Kolahgar et al., 2011). In such embryos, most epidermal cells undergo apoptosis, except in an approximately ten-cell-wide band of dorsal cells, despite strong activation of JNK signaling there. It appears, therefore, that the protective mechanism acts over a broader domain than just within the leading edge (see Figures 1A and 1B for a diagram of the relevant region of the embryonic epidermis).
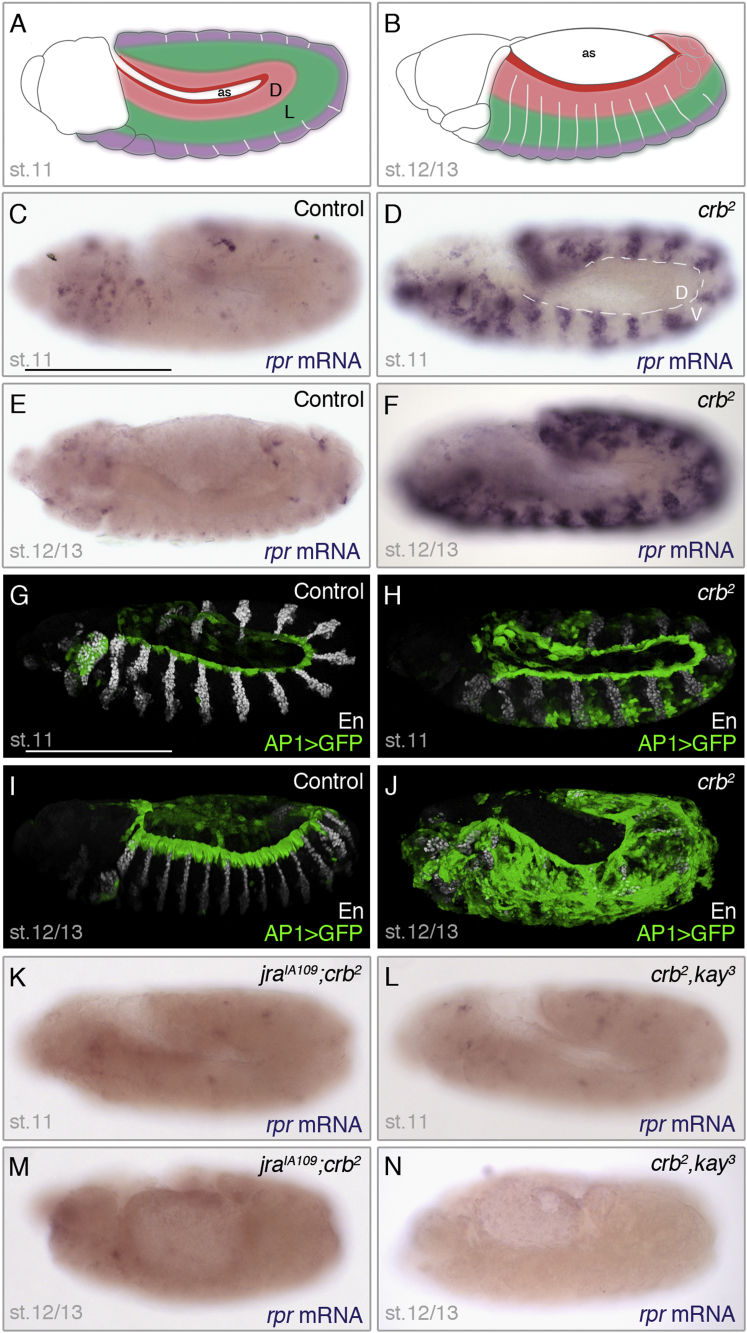
Figure 1.
Epithelial Disruption Triggers Canonical JNK Signaling, which, in Turn, Activates reaper Transcription in the Ventral and Lateral Epidermis of Drosophila Embryos
(A and B) Diagram of Drosophila embryos at stage (st.) 11 and stage 12/13, indicating the key epidermal domains: the dorsal (D) edge (deep red), the dorsal epidermis (faded red), the lateral (L) epidermis (green), and the ventral epidermis (purple). as, amnioserosa.
(C–F) Expression of reaper in control (crb2/+) and crumbs mutant (crb2/ crb2) embryos at stages 11 (C and D) and 12/13 (E and F), as indicated. In homozygous crumbs mutants, segmental upregulation is seen in the ventrolateral (V) epidermis, while in the dorsal (D) epidermis (inside white dotted lines), it remains silent. mRNA, messenger RNA.
(G–J) A fluorescent reporter of JNK activity (Chatterjee and Bohmann, 2012) is active at the edge of the dorsal epidermis of control embryos (crb2/+) and throughout the epidermis of homozygous crumbs mutant embryos (crb2/ crb2). Engrailed (En) immunoreactivity provides positional landmarks along the anterior-posterior axis. Embryonic stages are indicated.
(K–N) Expression of reaper is not activated in crumbs mutant embryos that also lack jra (jun) or kayak (fos). As positive controls, crumbs mutant embryos were stained in parallel (data not shown). Genotypes and stages are indicated.
Scale bar, 200 μm.
Most apoptosis in Drosophila requires the H99 locus (White et al., 1994), which comprises the three main proapoptotic genes: reaper, hid, and grim. Among these, reaper is the most likely mediator of the response to epithelial disruption since it is upregulated in crb mutant embryos in a pattern prefiguring that of caspase immunoreactivity (Kolahgar et al., 2011). Moreover, overexpression of Puckered, a phosphatase that inhibits JNK signaling prevents reaper upregulation, as well as apoptosis, in crb mutants (Kolahgar et al., 2011). These observations suggested that loss of apicobasal polarity triggers JNK signaling (through an unknown mechanism), which, in turn, causes reaper expression and, hence, apoptosis. However, in the dorsal epidermis, JNK signaling does not activate reaper expression. Here, we show the molecular mechanism underpinning such protection and thus explain how JNK can control cell migration without triggering apoptosis.
Results and Discussion
Activation of reaper by Canonical JNK Signaling Mediates Apoptosis in Response to Epithelial Disruption
In crb mutant embryos, reaper is strongly upregulated (Figures 1C–1F) in a pattern similar to that of apoptosis (Figures S1A and S1B available online), while the other two main proapoptotic genes, hid and grim, remain largely silent (Kolahgar et al., 2011)). No activated caspase immunoreactivity was detectable in rpr87 crb double mutant embryos (Figure S1C), confirming the essential role of reaper and highlighting the need to uncover the mechanisms that activate reaper expression following loss of apicobasal polarity. As suggested previously (Kolahgar et al., 2011), JNK signaling is likely involved. Indeed, JNK signaling, as measured with a transcriptional reporter (AP-1 > GFP) (Chatterjee and Bohmann, 2012) was strongly activated in crumbs mutant embryos (Figures 1G–1J; Movie S1). Moreover, little reaper transcription was detectable in crumbs embryos that also lack jra or kayak, which encode the two components of AP-1, Jun, and Fos (Figures 1K–1N; see Figures S1F–S1I for reaper expression in the single mutants), and this was associated with a near-absence of apoptosis, as reported by activated caspase-3 immunoreactivity (Figures S1D and S1E). Notably, JNK signaling does not seem to necessarily cause apoptosis. In the ventral epidermis, the patterns of reaper expression and apoptosis (highlighted with anti-activated caspase) appeared to mirror the early segmental activation of JNK signaling (compare Figure 1D with Figure S1B), suggesting a relatively straightforward, likely causal, relationship there. However, neither reaper expression nor apoptosis were significantly activated in the dorsal epidermis, even at the dorsal edge, where JNK signaling is particularly active both in wild-type and crumbs mutants. What is the mechanism that protects the dorsal epidermis from the proapoptotic effect of JNK?
Dpp Signaling Prevents JNK from Activating reaper Expression in the Dorsal Epidermis
One feature of the dorsal epidermis is that it is under the influence of Dpp, a member of the BMP family of secreted growth factors (Hamaratoglu et al., 2014). Indeed, phospho-Smad (p-Smad) immunoreactivity (a mark of Dpp signaling) (Tanimoto et al., 2000) was detectable in this region before and during the time when JNK is active (Figure 2A). Therefore, Dpp signaling could prevent JNK signaling from activating reaper expression both in the dorsal epidermis of crumbs mutant embryos and, physiologically, at the dorsal edge of wild-type embryos. This was tested in embryos expressing a Dpp RNA interference (RNAi)-encoding transgene (Supplemental Information) under the control of the ubiquitous tubulin-gal4 driver. This led to reduced signaling as indicated by the loss of p-Smad immunoreactivity from stage 11 onward (Figure 2B). In these embryos, a band of reaper transcription was observed at the dorsal edge (Figure 2C), where JNK is known to be activated in the wild-type (see Figure 1I). A similar result was seen in embryos lacking zygotic (but not maternal) activity of thickveins (tkv), which encodes an essential Dpp receptor (Nellen et al., 1994) (Figure 2D). Expression of reaper was also seen in the approximately ten-cell-wide dorsal region in tkv crumbs double mutants (as well as in the rest of the epidermis; Figure 2F), suggesting that Dpp signaling prevents reaper expression throughout the dorsal epidermis. Caspase immunoreactivity became detectable throughout the epidermis of tkv crumbs mutants (Figure S1J), consistent with the notion that repression of reaper expression by Dpp signaling translates into anti-apoptotic activity.
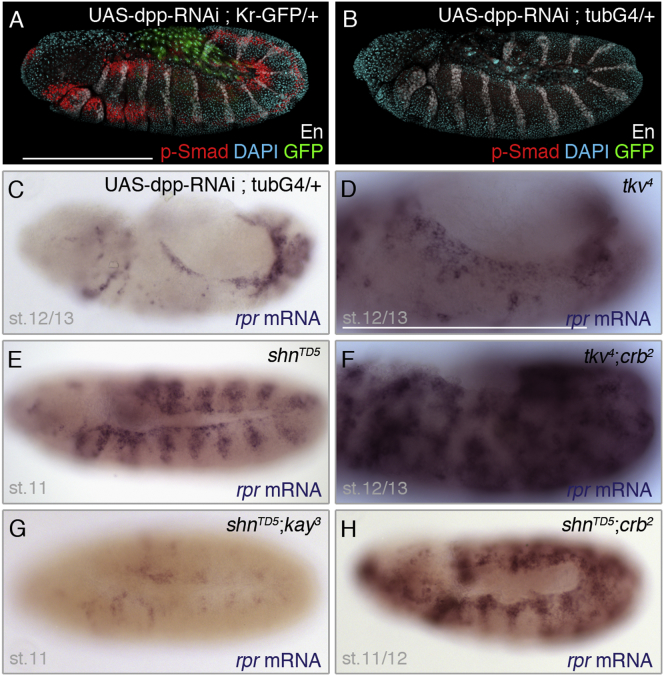
Figure 2.
Dpp Signaling Counteracts JNK-Dependent Activation of reaper via the Transcriptional Repressor Schnurri
(A and B) p-Smad immunoreactivity, seen in control embryos (A), is not detectable in embryos carrying both UAS-dpp[RNAi] and tubulin-Gal4 (B), confirming the effectiveness of this RNAi transgene. Anti-Engrailed and DAPI were used as morphological landmarks. En, Engrailed; st., stage.
(C) In embryos expressing UAS-dpp[RNAi] under the control of tubulin-Gal4, reaper expression becomes upregulated at the dorsal edge of the epidermis. Such embryos are often misshapen probably because of interference with the patterning activity of Dpp.
(D) Expression of reaper is also upregulated at the dorsal edge of homozygous thickveins (tkv) mutants.
(E) Upregulation of reaper in the dorsal and lateral epidermis of schnurri mutants. Expression can be seen along the whole dorsal edge but is segmental elsewhere. The basis of the segmental pattern is unknown.
(F) Upregulation of reaper throughout most of the epidermis of thickveins crumbs double mutants.
(G) No reaper upregulation is detected in schnurri kayak (fos) double mutants, demonstrating the requirement for canonical JNK signaling in reaper activation.
(H) Expression of reaper is upregulated throughout the epidermis of schnurri crumbs double mutants in a pattern that is roughly the sum of those seen in the single mutants.
Scale bar, 200 μm.
Schnurri Prevents reaper Transcription and Apoptosis
Dpp signaling is mediated by the Mad complex, which can activate or repress target genes depending on the sequence context and other cofactors (Affolter and Basler, 2007). The best characterized instance of repression by Dpp signaling is that of the brinker gene, which occurs via silencer elements where the Mad complex recruits the corepressor Schnurri (Affolter and Basler, 2007, Pyrowolakis et al., 2004). We therefore asked if Schnurri could mediate the repression of reaper by Dpp. Indeed, reaper transcription was upregulated at the dorsal edge of schnurri mutant embryos (Figure 2E; Figures S1O–S1R). In these embryos, reaper expression was also seen to extend segmentally in the lateral region, a feature not seen in Dpp-RNAi-expressing embryos (compare Figure 2E with Figure 2C), perhaps because Schnurri also has Dpp-independent activity, as suggested by (Kelsey et al., 2012). In any case, the reaper-repressive role of Schnurri in the dorsal epidermis was confirmed in schnurri crumbs double mutants, where reaper transcription was strongly upregulated in both the dorsal and ventral regions (Figure 2H). No reaper upregulation was seen in schnurri kayak (fos) double mutants (Figure 2G), showing that JNK signaling is an essential positive input to reaper transcription at the dorsal edge, as well as in segmentally repeated lateral domains, where the JNK sensor appears not to be sufficiently sensitive to detect activity. It is worth noting that staining of schnurri and schnurri crumbs embryos with anti-activated caspase confirmed that the repression of reaper transcription by Schnurri is needed to suppress apoptosis (Figures S1K and S1L). As AP-1 and Schnurri are both transcriptional regulators, we next sought to address whether the opposing influences of Schnurri and JNK signaling converge directly on the reaper promoter.
Binding Sites Upstream of the reaper Promoter Integrate the Effects of Dpp and JNK
The promoter region of brinker that mediates Schnurri-dependent repression has been extensively characterized, and mutation analysis identified an essential 16-base-pair (bp) repressor sequence (Pyrowolakis et al., 2004). Similar elements are found at ∼350 positions in the Drosophila genome, defining a consensus sequence: GRCGNCNNNNNGTCTG (Pyrowolakis et al., 2004). Two related sites were identified upstream of reaper, in a region that is conserved in the 12 sequenced Drosophila species. In all these species, the proximal site (SEp) is flanked on either side by a predicted AP-1-binding site, making it a potential regulatory element. Because SEp is not an exact match to the consensus Schnurri binding site, we used an electrophoretic mobility shift assay (EMSA) to test whether it is recognized by Schnurri, using the previously characterized site from the brinker gene (cSE) (Pyrowolakis et al., 2004) as a positive control. Recombinant Schnurri protein induced a supershift of the Mad-Medea-DNA complex in both cases, although to a lesser extent with SEp than with cSE (Figure S2A). Thus, we conclude that SEp is recognized by Schnurri/Mad/Medea and could therefore mediate the repressive influence of Dpp on reaper expression. By extension, the module comprising SEp and the two putative AP-1 sites could integrate the influence of Dpp and JNK signaling on reaper expression. To test this hypothesis in vivo, we made a reporter construct comprising 5.5 kb of sequence including this module and the basal reaper promoter, upstream of a GFP complementary DNA (Figure 3A). This reporter (rpr-GFP) and the variants described later were introduced by PhiC31-mediated integration at the same genomic location to allow comparison without confounding influence from position effects. The wild-type reporter was essentially silent in wild-type embryos (Figure 3B), as expected, since reaper expression is barely detectable during normal embryogenesis. By contrast, in crumbs mutants, rpr-GFP became segmentally upregulated in the ventrolateral—but not dorsal—epidermis (double-headed arrow in Figure 3C), thus mirroring the activity of the endogenous reaper gene in this background (compare to Figure 1F). Critically, like the endogenous reaper gene, this reporter became active in the dorsal epidermis of schnurri mutants (Figure 3D). Such dorsal expression was segmentally modulated (see Figure S2B for GFP staining alone), resembling the pattern of endogenous reaper transcription in schnurri mutants (Figure 2E). Also, like endogenous reaper, the reporter was widely and strongly activated in the epidermis of schnurri crumbs double mutants (Figure 3E). These observations suggest that Schnurri mediates repression of the reporter. To test the contribution of the predicted Schnurri binding site, a mutation was introduced in the reporter (ATCGTCTCGCCGTCTG → ATCGTCTCGCTTTCTG), thus creating rpr[ΔShn]-GFP (Figure 3A). This mutation was found to abrogate formation of the Mad-Medea-DNA complex in vitro (see SEm in Figure S2A), suggesting that rpr[ΔShn]-GFP would no longer be subject to repression by Schnurri. Indeed, this transgene became activated in wild-type embryos in the dorsal epidermis (Figure 3F). One must point out that this dorsal activity of rpr[ΔShn]-GFP did not appear before stage 13, 1–2 hr later than the appearance of rpr-GFP in schnurri mutants (compare Figure 3F to Figure 3D). Also, unlike rpr-GFP in schnurri mutants, rpr[ΔShn]-GFP was active in segmentally repeated ventral domains at stage 13. Despite these differences (see further discussion in the legend of Figure S2), the upregulation of rpr-GFP in schnurri mutants and the expression of rpr[ΔShn]-GFP in the dorsal epidermis of otherwise wild-type embryos (where rpr-GFP is silent) are consistent with the notion that Schnurri represses reaper expression in the dorsal epidermis, thus allowing JNK signaling to control epithelial migration without triggering apoptosis.
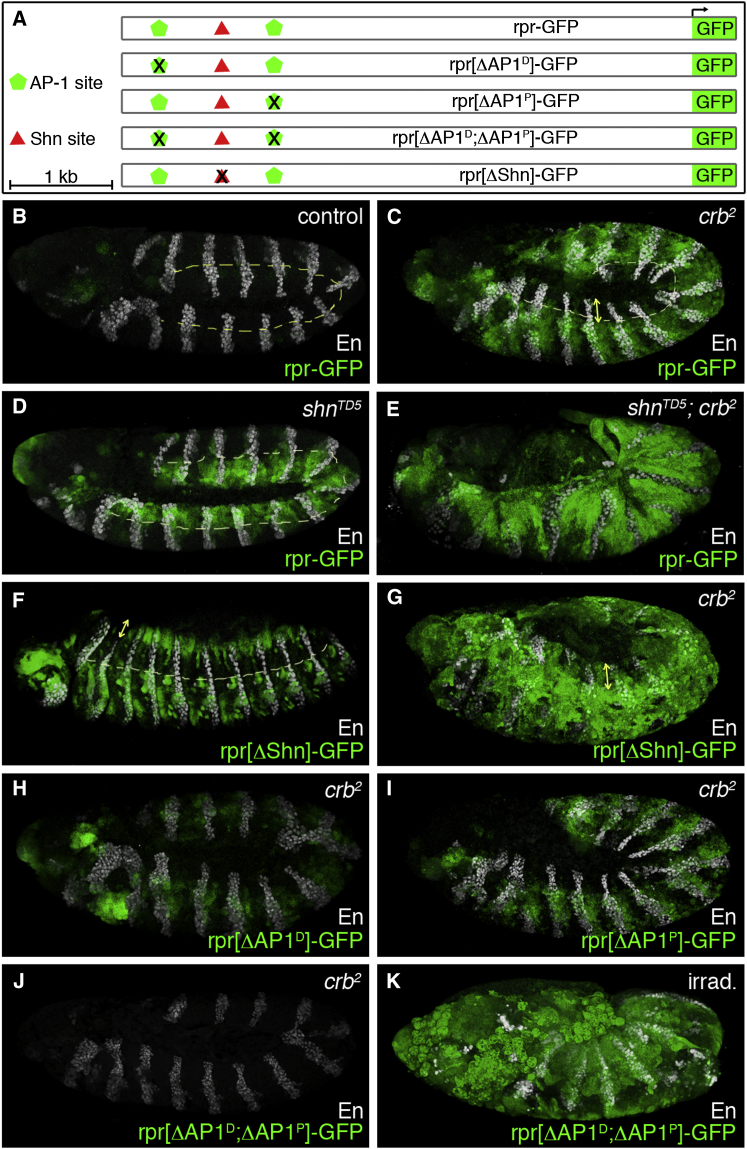
Figure 3.
The reaper Promoter Integrates Inputs from JNK and Dpp Signaling
(A) Diagram of the reporter constructs that were tested in transgenic embryos; 5.5 kb of the reaper promoter were used to create rpr-GFP. Predicted binding sites for Schnurri or AP-1 were mutated to generate the variants listed. Details of the mutations are indicated in the text.
(B) The unmutated reporter (rpr-GFP) is almost silent in wild-type embryos. En, Engrailed.
(C) In crumbs mutant embryos, the same reporter is active in the ventrolateral, but not the dorsal (double-headed arrow), epidermis.
(D) By contrast, in schnurri mutants, rpr-GFP becomes active in the dorsal epidermis, suggesting that Schnurri is an essential repressive factor.
(E) Embryos lacking both crumbs and schnurri upregulate rpr-GFP throughout much of the epidermis.
(F) Mutation of the predicted Schnurri binding site leads to upregulation of the reporter in the dorsal epidermis wild-type embryos at stage 13.
(G) In crumbs mutant embryos, rpr[ΔShn]-GFP upregulation is seen in the dorsal epidermis (double-headed arrow) as well as in the ventrolateral epidermis, consistent with the notion that Schnurri contributes to preventing reaper expression in the dorsal epidermis of crumbs embryos.
(H–J) Upregulation of the reaper reporter in crumbs mutants requires the two predicted AP-1 binding sites. Deletion of either site leads to reduced expression while the double mutant reporter (rpr[ΔAP1D; ΔAP1P]-GFP) is silent.
(K) Gamma-irradiated embryos carrying the rpr[ΔAP1D; ΔAP1P]-GFP transgene express GFP throughout, showing that this reporter is functional.
All embryos are shown at stage 11 except for (F), which shows a stage 12–13 embryo. Engrailed (En), shown in white, provides an indication of the embryos’ overall morphology.
We next assessed the function of the two predicted AP-1 binding sites by mutating them (TGACTCATA → TGACATTTA) (Chatterjee and Bohmann, 2012) individually or as a pair in the GFP reporter (Figure 3A) and assessing their activity in the ventral epidermis of crumbs embryos, where the wild-type reporter is strongly activated. Mutating one or the other site reduced activation, while the double mutant reporter (rpr[ΔAP1P;ΔAP1D]-GFP) had no detectable activity in crumbs mutants (Figures 3H–3J; wild-type background in Figure S2E). This reporter is nevertheless functional. It was activated by irradiation (Figure 3K), consistent with the presence of a p53-response element previously shown to mediate the response to irradiation (Brodsky et al., 2000). It is worth pointing out here that reaper expression was still activated in crb p535A-1-4 double mutants (P.F. Langton and J.-P.V., unpublished data), indicating that the response to loss of epithelial integrity does not require p53. Overall, our results show that the two predicted AP-1 binding sites contribute redundantly to reaper upregulation in crumbs mutants. They also show that JNK signaling acts directly on the reaper promoter and not via a relay mechanism. We conclude that a small regulatory module allows JNK to trigger apoptosis, except in dorsal cells that are protected by Dpp signaling.
Schnurri Ensures the Survival of Dorsal Edge Cells during Dorsal Closure
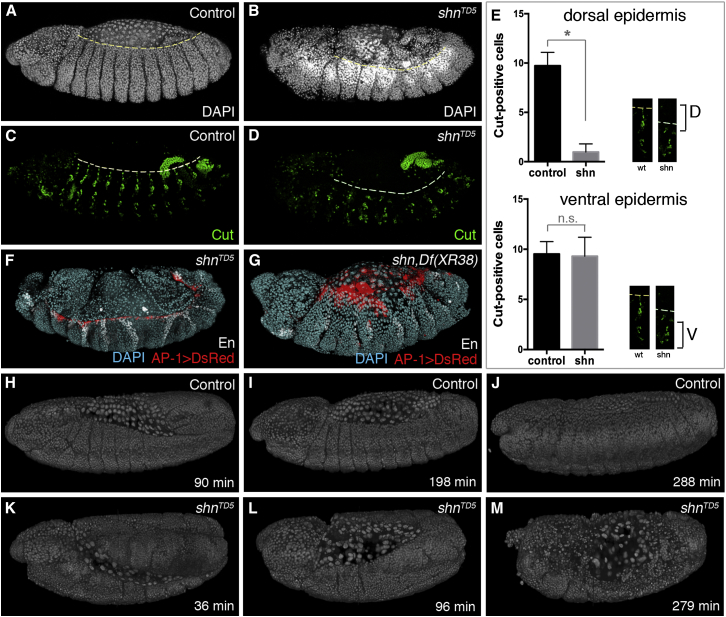
JNK and Dpp signaling have extensively been shown to orchestrate dorsal closure. So far, attention has been focused on the role of these pathways in triggering the cell shape changes required for the dorsal epidermis to spread over the amnioserosa and meet at the dorsal midline (Fernández et al., 2007, Homsy et al., 2006, Riesgo-Escovar et al., 1996). Our results suggest an additional role for Dpp signaling during dorsal closure, namely, to ensure the survival of leading edge cells. We propose that such a protective mechanism is needed because of the proapoptotic influence of JNK signaling. Accordingly, the “dorsal open phenotype” of schnurri mutants (and possibly other Dpp pathway mutants) would not only be caused by the failure of dorsal edge cells to migrate but also by their reduced survival. To evaluate the contribution of the latter, we assessed the extent of tissue loss in fixed and live schnurri mutant embryos, which, as previously reported (Arora et al., 1995, Grieder et al., 1995, Staehling-Hampton et al., 1995), do not undergo dorsal closure. Cursory observation suggests that, in schnurri mutant embryos, the dorsal hole that appears at the end of germband retraction gapes open over time (Figures 4A and 4B; Figures S1Q and S1R). This was confirmed by confocal live imaging of schnurri mutants carrying spider-GFP as a marker of cell outlines (Figure S3D; Movie S2). To test if gaping of the dorsal hole could be due to tissue shrinkage, stage ∼13 schnurri mutant embryos were stained with anti-Cut, which marks dorsal and ventral clusters of peripheral neurons along the D-V axis (Figures 4A–4D; Figures S3A–S3C), and the number of Cut-positive cells in each cluster was counted. While cell number in the dorsal cluster remained constant in wild-type embryos, it decreased significantly in schnurri mutants (Figure 4E). The number of ventral cells was relatively unaffected in both genotypes. It appears, therefore, that the dorsal epidermis is preferentially eliminated prior to this stage. Next, we used multiview light-sheet microscopy (MuVi-SPIM; Krzic et al., 2012) to achieve in toto imaging of schnurri mutants and control embryos carrying histone-red fluorescent protein (histone-RFP) (Figures 4H–4M). In schnurri mutants, cell debris could be seen around the dorsal edge, and many macrophages scurried around (Movies S3 and S4). Moreover, the number of epidermal nuclei became reduced, compared to that in control embryos (Figure S4). These observations confirm that, during stages 12–14, the dorsal epidermis of schnurri mutants progressively shrinks (Figures S4A–S4I), likely by apoptosis (see also Figures S1K, S1Q, and S1R). Consistent with the involvement of apoptosis, the dorsal hole did not appear to gape open in schnurri mutants that also lacked reaper (Figure 4G), even though these embryos failed to complete dorsal closure. As shown in Figure 4H, the JNK reporter remains active throughout tissue shrinkage (Figures 4F–4G), suggesting that, as dorsal edge cells are eliminated, adjacent epidermal cells activate JNK. Taken together, our results suggest that the dorsal open phenotype of schnurri mutants is a combined consequence of tissue loss by apoptosis and lack of cell migration over the amnioserosa.
Figure 4.
Dorsal Closure and Epithelial Loss in Wild-Type and schnurri Mutant Embryos
(A and B) Homozygous schnurri mutant embryos fail to achieve dorsal closure. A heterozygous embryo of similar stage is shown as control.
(C and D) The reduced number of Cut-positive cells in the dorsal cluster of schnurri mutant embryos suggests that the dorsal epidermis shrinks during the time when dorsal closure takes place in control embryos.
(E) Number of Cut-positive cells in the dorsal (D) and ventral (V) clusters of schnurri homozygotes and control heterozygotes at stage ∼13. A significant loss of cells is seen in the dorsal cluster (Student’s t test, ∗p < 0.0001). WT, wild-type; n.s., not significant. Error bars indicate SEM.
(F and G) schnurri and schnurri reaper mutant embryos carrying the JNK reporter. Gaping of dorsal hole is more pronounced in schnurri mutant (F) than in schnurri reaper mutant (G). En, Engrailed.
(H–M) Still images from MuVi-SPIM recordings (before, during, and after closure). Approximate times from the beginning of germband retraction are shown to allow comparison between the mutant and control samples. Control (H–J) and schnurri mutant (K–M) embryos carrying histone-RFP as a nuclear marker are shown. While dorsal cells migrate over the amnioserosa in the wild-type, these cells are progressively lost in schnurri mutants.
Most functions of Dpp signaling are achieved through Schnurri-dependent repression of the transcriptional repressor Brinker. However, as we have shown, inhibition of reaper by Schnurri is direct and, hence, likely Brinker independent. By contrast, the migration of dorsal edge cells is mediated by Brinker repression, since schnurri brinker double mutant larvae have a sealed dorsal midline (Marty et al., 2000, Torres-Vazquez et al., 2001). It is interesting that the dorsal epidermis of these double mutants is much reduced in surface area compared to that of wild-type embryos or brinker single mutant embryos, which have expanded dorsal fates (in Torres-Vazquez et al., 2001, compare Figure 2D to Figures 2A and 2B). We suggest that schnurri brinker double mutant embryos complete dorsal closure despite tissue loss, perhaps because a sufficient number of cells are able to migrate before undergoing apoptosis. To assess directly whether Brinker has any impact on reaper expression, we performed gain- and loss-of-function experiments (Figures S3E–S3I). No ectopic reaper expression was seen in embryos lacking or overexpressing brinker (Figures S3G and S3H). Moreover, Brinker overexpression did not prevent reaper expression in crumbs mutant embryos (Figures S3E and S3F), while overexpressing Schnurri did (Figures S3E and S3I). We conclude that Brinker does not affect reaper expression and that Schnurri contributes to dorsal closure through two parallel routes: first, by repressing Brinker and, hence, allowing the derepression of genes regulating the cytoskeletal functions required for cell migration (Fernández et al., 2007, Homsy et al., 2006); and second, by repressing reaper, thus ensuring survival of the dorsal edge cells in the face of JNK’s proapoptotic pressure. Our results highlight the central role of Dpp and Schnurri in cell survival, extending observations on Dpp in imaginal discs (Adachi-Yamada et al., 1999, Gibson and Perrimon, 2005, Moreno et al., 2002, Shen and Dahmann, 2005) and TGFβ in vertebrates (Sabapathy et al., 1999, Taya et al., 1999).
Conclusions
In many contexts, JNK signaling leads to apoptosis, perhaps a remnant of this pathway’s ancestral function in stress response (Ríos-Barrera and Riesgo-Escovar, 2013). JNK signaling may have started to regulate cytoskeletal functions in multicellular organisms to facilitate delamination or extrusion of defective cells. It is conceivable that such a regulatory relation might have subsequently been coopted to control other migratory behaviors such as those required for dorsal closure. Evidently, this would have necessitated coevolution of a protective, anti-apoptotic mechanism. At the dorsal edge of the epidermis, this is mediated by Dpp, which, intriguingly, is itself under the control of JNK signaling. Therefore, under the right circumstances, JNK contributes to the mechanism that counteracts its own AP-1-dependent proapoptotic pressure. The outcome of the regulatory network linking JNK and Dpp to reaper must be finely balanced, since a mild increase in JNK signaling (e.g., in puckered heterozygous embryos) triggers reaper expression (Kolahgar et al., 2011). Unlike the ventral epidermis, the dorsal epidermis seems prone to expressing Dpp in response to JNK, perhaps because of earlier expression in this region or through the action of additional regulators. This is likely to ensure the survival of dorsal edge cells during their migration. As we have shown, the opposing influences of Dpp and JNK are played out within the regulatory region of reaper. The anti-apoptotic activity of Dpp is mediated by Schnurri, a protein that could have more general anti-apoptotic activity since mammalian Schnurri has been shown to dampen cell death during T cell development (Staton et al., 2011). The function of this or similar regulatory modules in other tissues, contexts, and models can thus yield a broader understanding of the balance between apoptosis and survival at the intersection of signaling pathways.
Experimental Procedures
Details on materials and methods can be found in the Supplemental Information. These include a full list of the Drosophila strains and antibodies as well as step-by-step staining protocols for immunofluorescence and in situ hybridization. For gamma irradiation, embryos were collected for 4 hr, aged a further 4 hr, and introduced in a gamma-cell irradiator for a 4,000 rad exposure (Nordstrom et al., 1996). The embryos were allowed to recover for 2 hr at 25°C before further analysis. The reaper reporter constructs were created by standard molecular biology with primers listed in the Supplemental Information. They were introduced into the Drosophila genome by PhiC31-mediated integration into PBac[yellow[+]-attP-9A] VK00027 (Bloomington Drosophila Stock Center # 9744, on chromosome III). EMSAs were largely performed as described elsewhere (Pyrowolakis et al., 2004). Live embryo imaging was performed either by classical confocal or MuVi-SPIM. For classical confocal microscopy, we used a Leica SP5 microscope equipped with a resonant scanner and a 20× (NA, 0.8) water immersion objective. For MuVi-SPIM, we used a custom-built set-up (two Nikon 10× 0.3-NA illumination objective lenses and two Nikon 25× 1.1-NA detection lenses) and protocols as described elsewhere (Krzic et al., 2012). The custom-modified Hamamatsu Flash 4 cameras were operated in the Lightsheet Readout Mode to reject scattered photons. Details on image processing and data visualization are provided in the Supplemental Information.
Acknowledgments
We thank the Bloomington Drosophila Stock Center for fly stocks, as well as Dirk Bohmann, Henri Jasper, Markus Affolter, and Kristin White for reagents and Alex Gould and Ingrid Poernbacher for discussions. We also thank Pedro Gaspar for help generating the reporters. Marvin Albert provided an early version of the Mercator projection routine. This work was funded by the UK’s Medical Research Council (U117584268 to J.-P.V.) and a Wellcome Trust PhD Fellowship to J.V.B. S.G. was funded by the EMBL Interdisciplinary Postdoctoral Programme under Marie Curie Actions. L.H. acknowledges support from the Center of Modeling and Simulation in the Biosciences of the University of Heidelberg. Work in G.P.’s lab was supported by the research training program GRK1104 and the Excellence Initiative of the German Federal and State Governments (EXC294).
Published: October 9, 2014
Footnotes
This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Supplemental Information includes Supplemental Experimental Procedures, four figures, and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2014.08.015.
Contributor Information
Jorge V. Beira, Email: jbeira@gmail.com.
Jean-Paul Vincent, Email: jvincen@nimr.mrc.ac.uk.
Supplemental Information
Activity of TRE-GFP, a reporter of JNK activity, in an otherwise wild-type embryo (left) or a crumbs mutant embryo (right) from stage 10/11 onward. In the wild type, GFP fluorescence can be seen at the dorsal edge during dorsal closure, while in the mutant, widespread fluorescence becomes detectable as development proceeds. In this and in Movie S2, embryos are oriented with anterior on the left and dorsal at the top.
Dorsal closure in embryos carrying spider-GFP to mark cell outlines (lateral view showing dorsal closure at the top). Dorsal closure proceeds normally in the control embryo (left, schnurri heterozygote) but is impaired in the schnurri mutant (right). Note progressive shrinkage of the dorsal epidermis in the schnurri mutant during this period.
Whole-embryo imaging (MuVi-SPIM) of a wild-type (control) followed by a shn mutant embryo (second half of the movie) carrying histone-RFP. Initially, eight different views, each rotated by 45°, are shown to highlight distinct viewpoints around the embryo, starting with a dorsal view (followed by lateral, ventral, lateral on subsequent views). Subsequent frames show development during and after dorsal closure. Anterior is to the center of the rosette made up by the eight views. The first half of the movie shows the wild-type (control) embryo, while the second half shows a shn mutant, first from the dorsal side and then every 45°. Observation over time and from several angles confirms the progressive loss of epithelial tissue from the dorsal edge, where some cell debris can be seen and where macrophages later congregate.
Control (top) and schnurri mutant (bottom) embryos at three representative time points (before, during, and after dorsal closure). Each embryo undergoes a 360° rotation around the anterior-posterior axis to allow viewing of the whole epidermis (generated from MuVi-SPIM data). This shows the progressive loss of epidermal cells in the mutant, while dorsal cells migrate normally over the amnioserosa in the wild-type.
References
- Adachi-Yamada T., Fujimura-Kamada K., Nishida Y., Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- Affolter M., Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat. Rev. Genet. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Arora K., Dai H., Kazuko S.G., Jamal J., O’Connor M.B., Letsou A., Warrior R. The Drosophila schnurri gene acts in the Dpp/TGF beta signaling pathway and encodes a transcription factor homologous to the human MBP family. Cell. 1995;81:781–790. doi: 10.1016/0092-8674(95)90539-1. [DOI] [PubMed] [Google Scholar]
- Bergmann A., Agapite J., McCall K., Steller H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell. 1998;95:331–341. doi: 10.1016/s0092-8674(00)81765-1. [DOI] [PubMed] [Google Scholar]
- Bogoyevitch M.A., Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky M.H., Nordstrom W., Tsang G., Kwan E., Rubin G.M., Abrams J.M. Drosophila p53 binds a damage response element at the reaper locus. Cell. 2000;101:103–113. doi: 10.1016/S0092-8674(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee N., Bohmann D. A versatile ΦC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS ONE. 2012;7:e34063. doi: 10.1371/journal.pone.0034063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. JNK-induced apoptosis, compensatory growth, and cancer stem cells. Cancer Res. 2012;72:379–386. doi: 10.1158/0008-5472.CAN-11-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smaele E., Zazzeroni F., Papa S., Nguyen D.U., Jin R., Jones J., Cong R., Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran D.N., Reddy E.P. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández B.G., Arias A.M., Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech. Dev. 2007;124:884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Gibson M.C., Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Glise B., Noselli S. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev. 1997;11:1738–1747. doi: 10.1101/gad.11.13.1738. [DOI] [PubMed] [Google Scholar]
- Grieder N.C., Nellen D., Burke R., Basler K., Affolter M. Schnurri is required for Drosophila Dpp signaling and encodes a zinc finger protein similar to the mammalian transcription factor PRDII-BF1. Cell. 1995;81:791–800. doi: 10.1016/0092-8674(95)90540-5. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F., Affolter M., Pyrowolakis G. Dpp/BMP signaling in flies: From molecules to biology. Semin. Cell Dev. Biol. 2014;32C:128–136. doi: 10.1016/j.semcdb.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Homsy J.G., Jasper H., Peralta X.G., Wu H., Kiehart D.P., Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev. Dyn. 2006;235:427–434. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- Hou X.S., Goldstein E.S., Perrimon N. Drosophila Jun relays the Jun amino-terminal kinase signal transduction pathway to the Decapentaplegic signal transduction pathway in regulating epithelial cell sheet movement. Genes Dev. 1997;11:1728–1737. doi: 10.1101/gad.11.13.1728. [DOI] [PubMed] [Google Scholar]
- Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis. 2009;14:1021–1028. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- Kelsey E.M., Luo X., Brückner K., Jasper H. Schnurri regulates hemocyte function to promote tissue recovery after DNA damage. J. Cell Sci. 2012;125:1393–1400. doi: 10.1242/jcs.095323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahgar G., Bardet P.-L., Langton P.F., Alexandre C., Vincent J.-P. Apical deficiency triggers JNK-dependent apoptosis in the embryonic epidermis of Drosophila. Development. 2011;138:3021–3031. doi: 10.1242/dev.059980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzic U., Gunther S., Saunders T.E., Streichan S.J., Hufnagel L. Multiview light-sheet microscope for rapid in toto imaging. Nat. Methods. 2012;9:730–733. doi: 10.1038/nmeth.2064. [DOI] [PubMed] [Google Scholar]
- Leppä S., Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Luo X., Puig O., Hyun J., Bohmann D., Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 2007;26:380–390. doi: 10.1038/sj.emboj.7601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty T., Müller B., Basler K., Affolter M. Schnurri mediates Dpp-dependent repression of brinker transcription. Nat. Cell Biol. 2000;2:745–749. doi: 10.1038/35036383. [DOI] [PubMed] [Google Scholar]
- McEwen D.G., Peifer M. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 2005;132:3935–3946. doi: 10.1242/dev.01949. [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler K., Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- Nellen D., Affolter M., Basler K. Receptor serine/threonine kinases implicated in the control of Drosophila body pattern by decapentaplegic. Cell. 1994;78:225–237. doi: 10.1016/0092-8674(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Nordstrom W., Chen P., Steller H., Abrams J.M. Activation of the reaper gene during ectopic cell killing in Drosophila. Dev. Biol. 1996;180:213–226. doi: 10.1006/dbio.1996.0296. [DOI] [PubMed] [Google Scholar]
- Papa S., Zazzeroni F., Bubici C., Jayawardena S., Alvarez K., Matsuda S., Nguyen D.U., Pham C.G., Nelsbach A.H., Melis T. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat. Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Pyrowolakis G., Hartmann B., Müller B., Basler K., Affolter M. A simple molecular complex mediates widespread BMP-induced repression during Drosophila development. Dev. Cell. 2004;7:229–240. doi: 10.1016/j.devcel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar J.R., Hafen E. Drosophila Jun kinase regulates expression of decapentaplegic via the ETS-domain protein Aop and the AP-1 transcription factor DJun during dorsal closure. Genes Dev. 1997;11:1717–1727. doi: 10.1101/gad.11.13.1717. [DOI] [PubMed] [Google Scholar]
- Riesgo-Escovar J.R., Jenni M., Fritz A., Hafen E. The Drosophila Jun-N-terminal kinase is required for cell morphogenesis but not for DJun-dependent cell fate specification in the eye. Genes Dev. 1996;10:2759–2768. doi: 10.1101/gad.10.21.2759. [DOI] [PubMed] [Google Scholar]
- Ríos-Barrera L.D., Riesgo-Escovar J.R. Regulating cell morphogenesis: the Drosophila Jun N-terminal kinase pathway. Genesis. 2013;51:147–162. doi: 10.1002/dvg.22354. [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Jochum W., Hochedlinger K., Chang L., Karin M., Wagner E.F. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech. Dev. 1999;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Shen J., Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K., Laughon A.S., Hoffmann F.M. A Drosophila protein related to the human zinc finger transcription factor PRDII/MBPI/HIV-EP1 is required for dpp signaling. Development. 1995;121:3393–3403. doi: 10.1242/dev.121.10.3393. [DOI] [PubMed] [Google Scholar]
- Staton T.L., Lazarevic V., Jones D.C., Lanser A.J., Takagi T., Ishii S., Glimcher L.H. Dampening of death pathways by schnurri-2 is essential for T-cell development. Nature. 2011;472:105–109. doi: 10.1038/nature09848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B.E., Perrimon N. Stress signaling in Drosophila. Oncogene. 1999;18:6172–6182. doi: 10.1038/sj.onc.1203125. [DOI] [PubMed] [Google Scholar]
- Tanimoto H., Itoh S., ten Dijke P., Tabata T. Hedgehog creates a gradient of DPP activity in Drosophila wing imaginal discs. Mol. Cell. 2000;5:59–71. doi: 10.1016/s1097-2765(00)80403-7. [DOI] [PubMed] [Google Scholar]
- Taya Y., O’Kane S., Ferguson M.W. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J., Park S., Warrior R., Arora K. The transcription factor Schnurri plays a dual role in mediating Dpp signaling during embryogenesis. Development. 2001;128:1657–1670. doi: 10.1242/dev.128.9.1657. [DOI] [PubMed] [Google Scholar]
- Weston C.R., Davis R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- White K., Grether M.E., Abrams J.M., Young L., Farrell K., Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activity of TRE-GFP, a reporter of JNK activity, in an otherwise wild-type embryo (left) or a crumbs mutant embryo (right) from stage 10/11 onward. In the wild type, GFP fluorescence can be seen at the dorsal edge during dorsal closure, while in the mutant, widespread fluorescence becomes detectable as development proceeds. In this and in Movie S2, embryos are oriented with anterior on the left and dorsal at the top.
Dorsal closure in embryos carrying spider-GFP to mark cell outlines (lateral view showing dorsal closure at the top). Dorsal closure proceeds normally in the control embryo (left, schnurri heterozygote) but is impaired in the schnurri mutant (right). Note progressive shrinkage of the dorsal epidermis in the schnurri mutant during this period.
Whole-embryo imaging (MuVi-SPIM) of a wild-type (control) followed by a shn mutant embryo (second half of the movie) carrying histone-RFP. Initially, eight different views, each rotated by 45°, are shown to highlight distinct viewpoints around the embryo, starting with a dorsal view (followed by lateral, ventral, lateral on subsequent views). Subsequent frames show development during and after dorsal closure. Anterior is to the center of the rosette made up by the eight views. The first half of the movie shows the wild-type (control) embryo, while the second half shows a shn mutant, first from the dorsal side and then every 45°. Observation over time and from several angles confirms the progressive loss of epithelial tissue from the dorsal edge, where some cell debris can be seen and where macrophages later congregate.
Control (top) and schnurri mutant (bottom) embryos at three representative time points (before, during, and after dorsal closure). Each embryo undergoes a 360° rotation around the anterior-posterior axis to allow viewing of the whole epidermis (generated from MuVi-SPIM data). This shows the progressive loss of epidermal cells in the mutant, while dorsal cells migrate normally over the amnioserosa in the wild-type.