Abstract
Objectives
To evaluate the first experience of real-time instantaneous wave–free ratio (iFR) measurement by clinicians.
Background
The iFR is a new vasodilator-free index of coronary stenosis severity, calculated as a trans-lesion pressure ratio during a specific period of baseline diastole, when distal resistance is lowest and stable. Because all previous studies have calculated iFR offline, the feasibility of real-time iFR measurement has never been assessed.
Methods
Three hundred ninety-two stenoses with angiographically intermediate stenoses were included in this multicenter international analysis. Instantaneous wave–free ratio and fractional flow reserve (FFR) were performed in real time on commercially available consoles. The classification agreement of coronary stenoses between iFR and FFR was calculated.
Results
Instantaneous wave–free ratio and FFR maintain a close level of diagnostic agreement when both are measured by clinicians in real time (for a clinical 0.80 FFR cutoff: area under the receiver operating characteristic curve [ROCAUC] 0.87, classification match 80%, and optimal iFR cutoff 0.90; for a ischemic 0.75 FFR cutoff: iFR ROCAUC 0.90, classification match 88%, and optimal iFR cutoff 0.85; if the FFR 0.75-0.80 gray zone is accounted for: ROCAUC 0.93, classification match 92%). When iFR and FFR are evaluated together in a hybrid decision-making strategy, 61% of the population is spared from vasodilator while maintaining a 94% overall agreement with FFR lesion classification.
Conclusion
When measured in real time, iFR maintains the close relationship to FFR reported in offline studies. These findings confirm the feasibility and reliability of real-time iFR calculation by clinicians.
Graphical Abstract
The instantaneous wave–free ratio (iFR) is a recently proposed index of coronary disease severity, which uses a trans-lesional pressure ratio as a measure of functional stenosis severity. It can be calculated using conventional pressure guide wires and differs fundamentally from fractional flow reserve (FFR)1 because it does not require vasodilators such as adenosine for its calculation.
The relationship between iFR and FFR has been extensively evaluated in more than 2,000 stenoses,[1], [2], [3], [4] and their agreement in lesion classification ranges from 80% to 90%, depending on whether the comparison is made in clinical populations, with predominantly intermediate lesions, or in samples with more severe stenoses.2 When compared with independent arbiters of stenosis severity, iFR and FFR have demonstrated equal diagnostic agreement against invasive flow[5], [6] and myocardial perfusion imaging.7 Also, offline studies consistently demonstrated 0.89 to 0.90 as the optimal iFR cutoff to match an FFR of 0.80.[2], [3], [8]
To date, however, in all iFR-FFR studies, pressure data and iFR calculation have been processed offline in a core laboratory, after procedural termination and appropriate data extraction. Although necessary for any new technology during its early development, this methodology does not reflect the future application of iFR in clinical practice, which will be performed by clinicians, using appropriate software installed in hemodynamic consoles. It is not known whether these practical aspects could affect the performance of iFR.
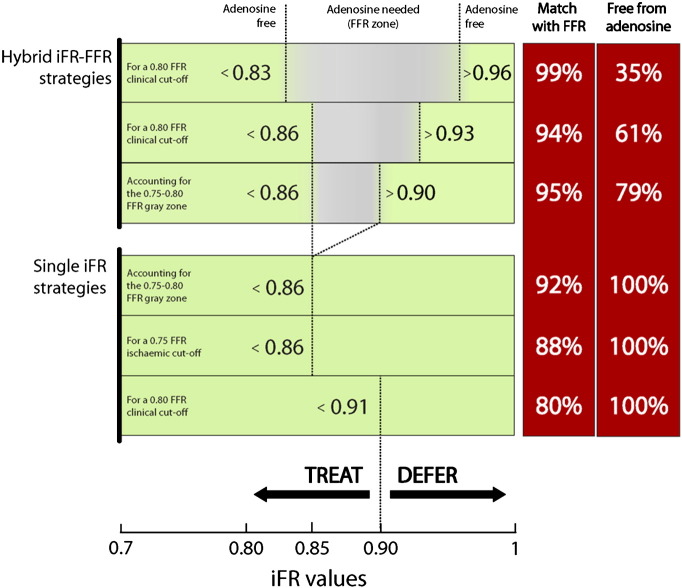
In this study we explored important aspects of the relationship between iFR and FFR, when both indices are measured in real time by clinicians. First, using a clinical FFR cutoff of 0.80 as a reference, we evaluated whether the diagnostic performance and optimal cutoff of iFR are maintained, when compared with offline studies. Second, we extended the analysis to the original 0.75 ischemic FFR cutoff and explored the performance of real-time iFR to match such classification of stenoses. Finally, we aimed to validate with real-time measurements the previously reported iFR-FFR hybrid strategy, using a predefined deferral iFR cutoff of >0.93 and a predefined treatment iFR cutoff of <0.86.9
Methods
Study population
Instantaneous wave–free ratio and FFR were measured from hemodynamic consoles in 16 centers in Europe, Asia, and Africa. The study included 392 stenoses from 313 consecutive patients who, as part of clinical investigation, required functional intracoronary assessment with pressure guide wires.
Hemodynamic data collection and analysis
Cardiac catheterization was performed according to standard practice. Unfractionated intravenous heparin (5000 IU) and 300 to 600 μg intracoronary nitrates were given at the start of the procedure. Acquisition of physiological data for FFR calculation was performed according to conventional practice10 using commercially available FFR systems (S5i and Prestige pressure guide wire; Volcano Corporation, San Diego, California). The method to induce pharamacologic hyperemia varied according to conventional clinical practice at each center. Adenosine (or adenosine triphosphate) was used for induction of hyperemia: in 39% of the cases, it was administered via a central line, with doses ranging from 140 to 180 μg kg−1 min−1 (median 140 μg kg−1 min−1); in 61% of the cases, the intracoronary route was used with a median dose of 60 μg (interquartile range 36-240 μg). Each reported iFR value and its FFR counterpart were obtained directly from the hardware console. Instantaneous wave–free ratio was calculated using software embedded onto the hemodynamic consoles, which uses proprietary iFR algorithms acting on electrocardiogram (ECG)-gated, time-aligned pressure traces, as previously described.1 Instantaneous wave–free ratio was automatically calculated as a ratio of distal (Pd) to proximal (Pa) coronary pressures at the baseline iFR window. Fractional flow reserve was automatically calculated as the ratio of whole-cycle Pd/Pa during hyperemia. Anatomical severity of coronary stenoses was measured using quantitative coronary angiography.
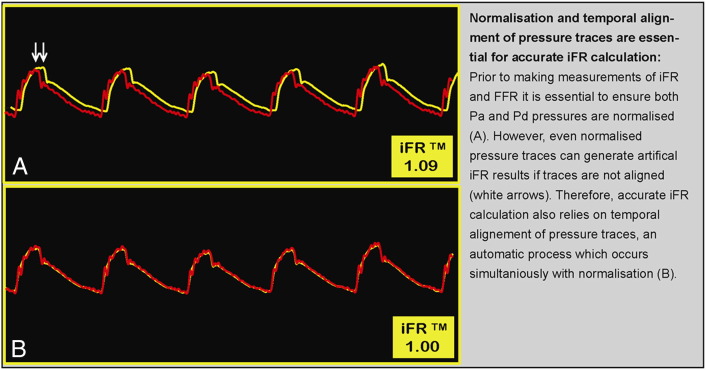
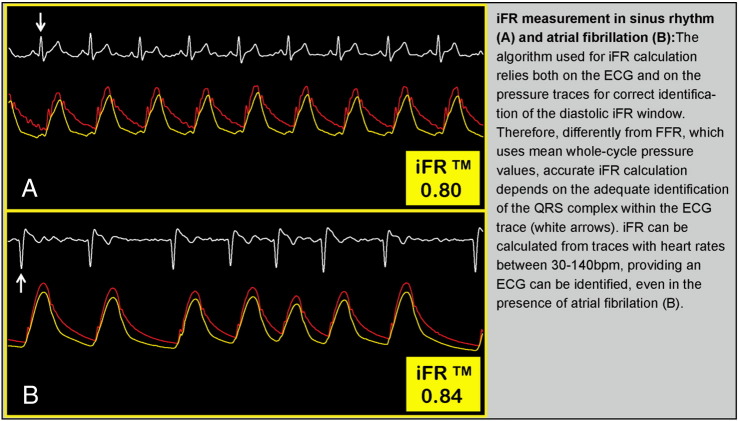
Prior to iFR and FFR measurements, hemodynamic consoles ensure that the pressure data are appropriately calibrated. These include steps required for both FFR and iFR calculation, such as ensuring that catheter (Pa) and wire (Pd) pressures are equal or normalized before wire insertion. In addition, a specific step is needed before iFR measurement, which is the adjustment of temporal delays between Pa and Pd signals. This essential process occurs automatically and simultaneously with pressure normalization (Figure 1). Figure 2 demonstrates the importance of ECG identification and the reliability of iFR calculation in sinus rhythm and atrial fibrillation.
Figure 1.
Pressure normalization, temporal alignment, and iFR calculation using the hemodynamic console.
Figure 2.
Importance of ECG detection for accurate iFR measurement.
Statistical analysis
The classification agreement (and sensitivity, specificity, negative predictive value, and positive predictive value) between iFR and FFR as well the area under the receiver operating characteristic curves (ROCAUC) were calculated for 2 different FFR cutoffs. First, a 0.80 clinical FFR cutoff (as per FAME, FAME II trials, and current clinical guidelines) was used (FFR or iFR ≤0.8 as a reference test to define significant stenoses). For this clinical FFR cutoff, we established a prespecified iFR cutoff of 0.90 and evaluated whether it would also represent the optimal cutoff by ROC analysis (defined as the best sum of sensitivity and specificity). Second, the diagnostic performance of iFR was evaluated for a 0.75 ischemic FFR cutoff, as per original validation studies and DEFER[11], [12] (FFR or iFR ≤0.75 as a reference test to define significant stenoses). Finally, the diagnostic performance of iFR was explored when allowing for the 0.75 to 0.80 FFR gray zone (zone of values which, according to DEFER and FAME/FAME II studies, is both safe to defer and treat stenoses). Comparisons between proportions (classification agreement between iFR and FFR) observed in this study with previous offline data sets were made with the χ2 test for homogeneity of proportions. Areas under the receiver operating characteristic curve were compared using the method described by DeLong et al13 in STATA, version 11 (StataCorp, College Station, Texas). Data are expressed as mean ± SD. A P value of <.05 was considered statistically significant.
Hybrid iFR-FFR analysis
The real-time iFR and FFR values reported in this sample were evaluated in a hybrid decision-making strategy, according to the methodology previously described.9 We aimed to prospectively apply the following decision-making arms into this sample: when iFR >0.93, stenoses would be deferred; when iFR <0.86, stenoses would be treated; when iFR would fall between 0.86 and 0.93, FFR would be calculated. For such strategy, we evaluated the overall agreement in stenoses classification with an FFR-only strategy and the proportion of stenoses spared from vasodilator administration.
Funding sources
Dr Petraco (FS/11/46/28861) and Dr. J.E. Davies (FS/05/006) are British Heart Foundation fellows. Dr Sen (G1000357) and Dr Nijjer (G1100443) are Medical Research Council fellows. Dr Di Mario is a senior National Institute for Health Research investigator. No other extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Sample characteristics
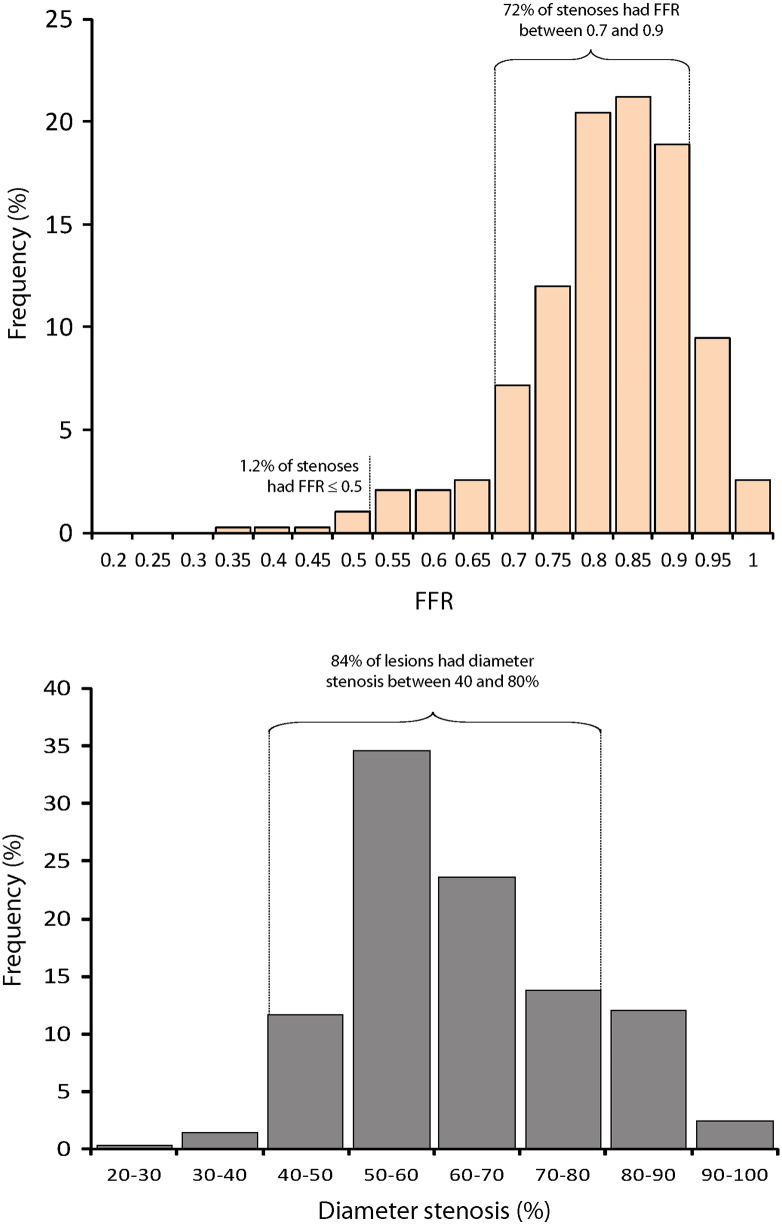
The 392 stenoses (from 313 patients) included in this study demonstrated a unimodal distribution of FFR and diameter stenosis values. Population demographic and angiographic data are presented in Table. Fractional flow reserve and iFR were measured in all attempted cases. Mean FFR was 0.82 ± 0.10, and mean iFR was 0.89 ± 0.11. This sample was formed predominantly by physiologically intermediate lesions, with 71% of stenoses falling between FFR 0.70 and 0.90. The proportion of stenoses with FFR values lower than 0.80, 0.75, 0.60, and 0.50, respectively, was 39%, 18%, 4.6%, and 1.3%. Mean percentage diameter stenosis was 56% ± 13%, with 84% of lesions falling within the 40% to 80% group. Figure 3 presents a histogram of FFR and diameter stenosis values.
Table.
Demographic and angiographic data
| No. of stenoses (patients) | 392 (313) |
| Age (y), mean ± SD | 67 ± 11 |
| Male % | 79 |
| Comorbidities (%) | |
| Hypertension | 74 |
| Hypercholesterolemia | 67 |
| Smoking history | 51 |
| Diabetes | 30 |
| Ejection fraction, mean ± SD | 58 ± 12 |
| Clinical presentation (%) | |
| Stable angina | 73 |
| Unstable angina (nonculprit vessel) | 27 |
| Coronary anatomy (%) | |
| Single-vessel CAD | 36 |
| Multivessel CAD | 63 |
| LAD | 66 |
| LCx | 10 |
| RCA | 14 |
| Other | 10 |
| Diameter stenosis (%), men ± SD | 56 ± 13 |
| Adenosine route (%) | |
| Intravenous | 39 |
| Intracoronary | 61 |
Abbreviations: CAD, Coronary artery disease; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery.
Figure 3.
Frequency distribution of FFR and percentage diameter stenosis values in the study.
Classification agreement between iFR and FFR
Using a clinical FFR cutoff of 0.80
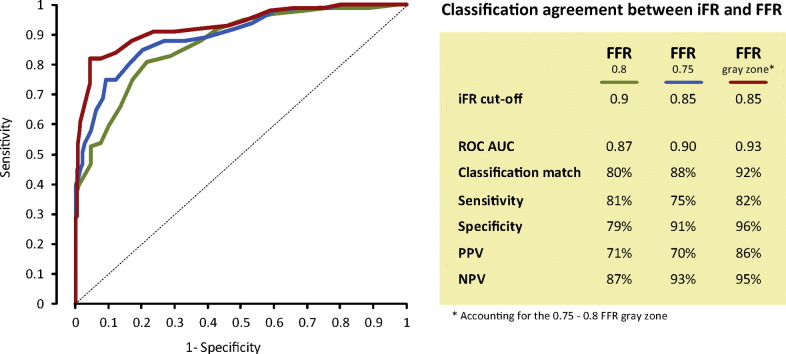
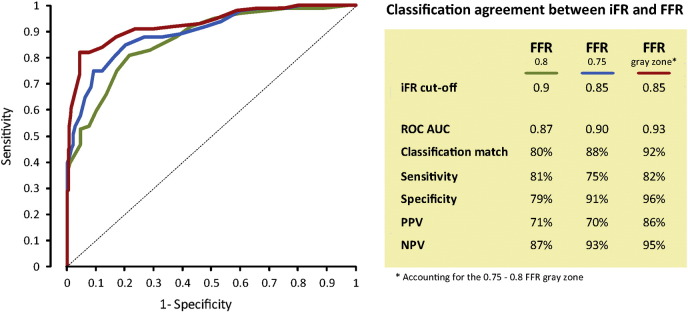
In this sample, the predefined iFR cutoff of 0.90 was also identified as the ROC-derived optimal cutoff, yielding a classification agreement with FFR of 80%. The ROCAUC for iFR to was 0.87 (CI 0.84-0.91). The diagnostic agreement between iFR and FFR when measured in real time (80%) was not different from previously reported offline studies performed in clinical samples (RESOLVE study8 80%, N = 1,593; ADVISE Registry2 study 80%, N = 339; and by Park et al3 82%, N = 238; P = .95 for all comparisons). The detailed diagnostic performance of iFR is summarized in Figure 4. For this clinical FFR cutoff, the diagnostic relationship between iFR and FFR remained close within the intermediate anatomical lesion range (iFR-FFR ROCAUC within 40%-80% diameter stenosis = 0.86 [0.83-0.90]).
Figure 4.
Diagnostic agreement between iFR and FFR.
Using a ischemic FFR cutoff of 0.75
For the ischemic FFR cutoff, classification match between iFR and FFR was 88% with an ROCAUC of 0.90 (CI 0.86-0.94). To match an FFR of 0.75, the ROC-derived optimal iFR cutoff was 0.85 (Figure 4). For this ischemic FFR cutoff, the diagnostic relationship between iFR and FFR also remained unchanged within the intermediate anatomical lesion range (iFR-FFR ROCAUC within 40%-80% diameter stenosis = 0.88 [0.85-0.91]).
When the FFR values falling into the 0.75 to 0.80 gray zone were considered equally safe to be classified as normal or abnormal, the ROCAUC increased to 0.93 (CI 0.90-0.96) and the classification agreement between iFR and FFR to 92%. Both ROCAUC and classification agreement were significantly higher when accounting for the FFR gray zone, when compared with single FFR cutoffs (P < .001 for comparisons against FFR 0.75 and FFR 0.80).
Hybrid iFR-FFR decision-making analysis
The previously reported iFR cutoffs of a hybrid iFR-FFR approach9 were also validated in this real-time iFR sample. Using a predefined deferral iFR cutoff of >0.93 and a predefined treatment iFR cutoff of <0.86 (and measuring FFR only in those lesions with iFR between 0.86 and 0.93) would generate a 94% overall agreement with an FFR classification of lesions, while sparing 61% of patients from adenosine, proportions that are not different from the previous offline report (Petraco et al,9 N = 577; P = .92). Figure 5 summarizes other possible iFR-FFR diagnostic strategies.
Figure 5.
Decision-making strategies of revascularization, using iFR only (bottom panel) and a hybrid iFR-FFR approach (top panel). FFR gray zone (0.75-0.80) refers to a region within which is known to be safe to defer and treat stenoses with equivalent clinical outcomes.
Discussion
In this study, we present the results of the first clinical application of real-time iFR measurements in patients undergoing invasive functional assessment of intermediate coronary stenoses. We found that (1) for a clinical FFR cutoff of 0.80, a predefined iFR value of 0.90 provides the optimal cutoff, with a classification match of 80%, similar to what has been reported in previous offline studies; (2) when the originally validated 0.75 ischemic FFR cutoff is used as a reference comparison, the agreement between iFR and FFR increases to 88% with the optimal iFR cutoff being 0.85; (3) when accounting for the known 0.75 to 0.80 FFR gray zone, the classification match between iFR and FFR increases to 93%; and (4) confirming previous reports,[8], [9] a hybrid decision-making strategy with iFR and FFR could spare 61% of patients from vasodilator, while maintaining 94% overall agreement with FFR classification of lesions.
Real-time iFR measurement in the catheterization laboratory is feasible
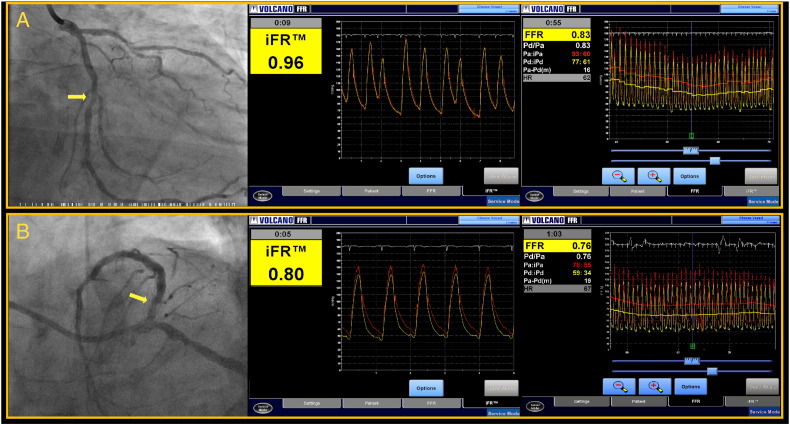
One potential limitation applicable to all studies that so far evaluated the relationship between iFR and FFR is the fact that all hemodynamic analyses were performed offline. This inevitable stage in the development of a new technology creates the theoretical possibility of expertise bias where only operators specifically trained in the offline analysis of hemodynamic traces, such as in a core laboratory, perform the analysis. We have found that when iFR is implemented in catheter laboratories around Europe, Africa, and Asia and the measurements are performed by clinicians who routinely perform functional assessment using pressure guide wires, the close agreement between iFR and FFR is maintained. Our results, therefore, demonstrate the feasibility of iFR use by the clinical community and the applicability of earlier offline core laboratory reports to clinical practice. Figure 6 shows 2 examples of lesion interrogation by iFR and FFR.
Figure 6.
Screenshots of measurements of iFR and FFR. A, An example of interrogation in the left circumflex artery (horizontal arrow), in which both iFR and FFR were negative, above their respective cutoffs of 0.90 and 0.80; revascularization was deferred. B, An example in which both iFR and FFR revealed a functionally significant stenosis in the proximal segment of the left anterior descending artery (oblique arrow); percutaneous revascularization was performed. Because iFR is performed without the need for vasodilator administration, the time of lesion interrogation is typically reduced to around 5 to 10 seconds, from 60 to 120 seconds for FFR.
Validation of the clinical iFR cutoff and match with FFR classification
In this study, when a clinical FFR cutoff of 0.80 was used as a reference comparison, a prespecified iFR value of 0.90 was also found to be the optimal cutoff identified by ROC, yielding a classification agreement of 80%, with an ROCAUC of 0.87. This comparison with an FFR of 0.80 is important because it reflects current clinical guidelines,14 is in line with FAME15 and FAME II studies,16 and validates with real-time measurements the results of all previous iFR-FFR studies in clinical populations.[2], [3], [8]
Performance of iFR to identify FFR-ischemic stenoses
Despite its clinical use for decision making, it must be remembered that FFR 0.80 is not an ischemia-derived FFR optimal cutoff. Studies that evaluated FFR against measures of myocardial perfusion more consistently identified FFR 0.74 to 0.75 as the optimal FFR cutoff.11 The change from FFR 0.75 to 0.80 was subsequently implemented in large clinical trials[15], [16] and was aimed to increase the negative predictive value of FFR-based decisions and avoid significant lesions being missed by a lower cutoff of FFR 0.75. Therefore, the original and extensively validated ischemic 0.75 FFR cutoff12 13 is also essential for new indices to be validated against, as it has been shown to provide the best match to previous perfusion modalities.[11], [14], [15] Indeed, in ADVISE in-practice when such a comparison was made, the agreement between iFR and FFR increased to 88% when an iFR cutoff of 0.85 was used. In addition, the finding of an iFR cutoff of 0.85 being equivalent to an FFR of 0.75 is very supportive to the ischemic iFR cutoff found in the CLARIFY study (iFR 0.86), which used invasive flow as a reference discriminator.5 Also, similarly to what has been observed with FFR, a higher iFR value of >0.90 increases the negative predictive value of iFR to 95% to exclude ischemic stenoses (FFR ≤ 0.75).
Therefore, across studies reported so far, in more than 2,000 patients, there appears to be a consistent ischemic iFR cutoff of 0.85 to 0.86, which matches an FFR 0.75, and a clinical iFR cutoff of iFR 0.89 to 0.90, which provides the best agreement with the clinically used FFR of 0.80. Reassuringly, similar to FFR, our findings confirm the presence of these 2 distinct iFR cutoffs (ischemic and clinical) when measurements are performed in real time, by interventionalists in the catheter laboratory.
The FFR 0.75 to 0.80 gray zone: safety implications for the development of new indices
Early studies that validated FFR as a new diagnostic method identified optimal cutoffs that varied slightly from 0.66, 0.72, 0.74, to 0.75.11 The validity of the 0.75 cutoff was subsequently confirmed in 2 landmark studies, which laid the foundations of FFR as a clinical tool. First, the multitesting study that compared FFR against 3 noninvasive functional methods17 undoubtedly demonstrated that no ischemia was detected when FFR fell between 0.75 and 0.80 and only 1 case when between 0.74 and 0.83. This safety was later translated into clinical outcomes with the results of the DEFER study,12 which documented a rate of major cardiac events of less than 0.6% per year for deferred stenoses with FFR values of ≥0.75.18 Recently, however, FAME and FAME II have also demonstrated the safety and prognostic importance of revascularizing lesions with FFR ≤0.80.[15], [16] Therefore, the 0.75 to 0.80 FFR zone is a region within which it is known to be equally safe to defer and treat stenoses, where ischemia is almost certainly absent but cannot be always excluded.
This DEFER-FAME gray zone not only is clinically relevant (as it permits clinicians to defer or treat stenoses with the same confidence)19 but also has implications to the development of new indices, which use the safety of FFR classification of lesions as a reference comparison. For instance, we have found that when this gray zone is taken into account, and either a DEFER or FAME approach is taken, iFR classification match with FFR increases to 92% with an ROCAUC of 0.93. This suggests that most of iFR-FFR disagreements are unlikely to be prognostic, as they predominantly fall in the FFR gray zone. Future trials with clinical outcomes will need to confirm this finding prospectively.
Hybrid approach confirms adenosine-sparing potential of iFR application
Until clinical outcome studies judge the merits of iFR as an independent diagnostic method, clinicians might prefer to maintain a higher magnitude (>90%) of classification agreement with FFR, given its established role to select lesions for revascularization. For this purpose, a hybrid decision-making strategy using both iFR and FFR can be applied. A hybrid analysis on this real-time iFR data set with prespecified cutoffs has reproduced the results reported previously.9 Our findings confirm the potential feasibility of using iFR and FFR together in a hybrid decision-making approach, in which 61% of patients could be spared from vasodilator, while maintaining the safety of a 94% match with FFR classification of lesions (Figure 5).
Study samples of patients undergoing invasive physiological assessment in clinical practice: how do they differ from large randomized clinical trials?
The present study also highlights an important feature of unselected clinical FFR cohorts: they are predominantly formed by intermediate FFR values. In the present sample, mean FFR was 0.82 ± 0.10, with 71% of stenoses falling within the 0.70 and 0.90 FFR range and only 20% ≤0.75 and 39% ≤0.80. Importantly, physiologically severe lesions appear extremely rare in samples in which FFR is used to guide clinical decisions, with only 4.6% having an FFR ≤0.6 and 1.3% ≤0.5. These figures are very similar to what has been previously reported in large independent clinical cohorts from Europe2 and Asia3 and reflect routine clinical application of FFR as recommended per guidelines.14 Importantly, they differ markedly from the distribution of FFR values of previous large FFR clinical trials. For instance, mean FFR of treated lesions in DEFER12 was 0.57 ± 0.16, while in FAME,15 it was 0.60 ± 0.14. Notably, in the medical arm of FAME II study,16 19% and 26% of the stenoses had FFR less than 0.50 and 0.60, respectively, which contrasts with the rarity of such severe lesions in clinical samples.
These differences between unselected clinical cohorts and studies in which patients are actively recruited by investigators are very relevant. First, they demonstrate that interventionalists have an excellent overall clinical judgement as to which stenoses require functional interrogation, as most clinically selected anatomically intermediate stenoses are also physiologically intermediate (Figure 3). Second, it appears that clinical populations such as found in this study provide the most conservative scenario with respect to the magnitude of agreement between iFR and FFR, because they are predominantly formed by intermediate FFR values, when disagreements are naturally higher.[2], [20] In populations with more severe lesions, such as in DEFER or FAME studies, classification agreement between iFR and a clinical FFR cutoff of 0.80 is expected to be higher, around 90%, as previously demonstrated.[1], [21]
Clinical implications of our findings
Routine clinical application of a vasodilator-free index such as iFR could potentially expand the benefits of physiology-guided revascularization to many more patients with coronary disease, as the need for vasodilator is one of the reasons for the low adoption of FFR.22 In South Africa, for example, 15 ampoules of a specific adenosine preparation are required for one intravenous infusion, at considerable cost. Similar restrictions occur in other countries, such as Russia, Turkey, and in Latin America, where papaverine is still frequently used as a hyperemic agent with its incumbent risks of serious cardiac arrhythmias.23 The costs and procedural delays associated with vasodilator administration (particularly long intravenous infusions, the recommended route for a complete vessel assessment) are particularly relevant to patients with multivessel disease, who might need 3-vessel interrogation. This group of patients is perhaps the one that benefits the most from invasive evaluation of intermediate stenoses,[15], [16] although it appears to be the most affected by the lack of its widespread adoption.
Future directions: the need for clinical trials
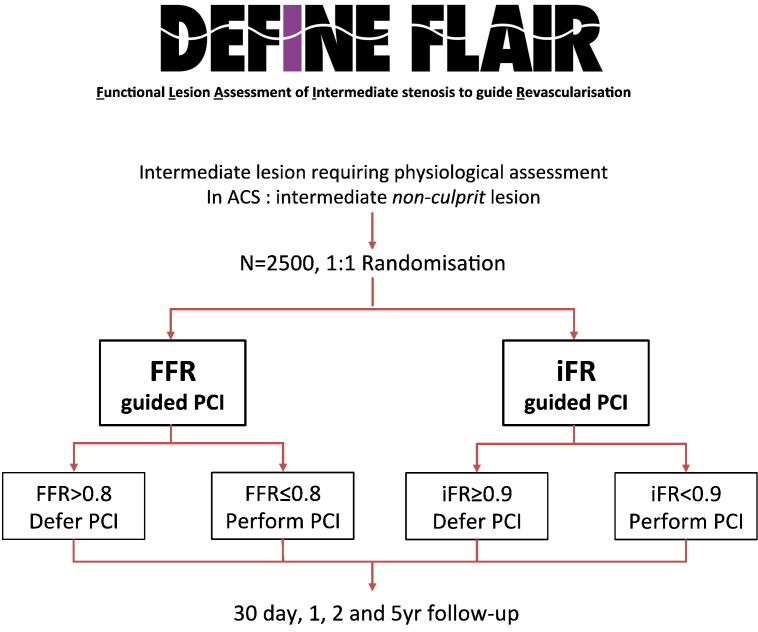
An accumulation of evidence supports iFR as an index of stenosis severity. However, clinical outcome data are required to further advance the global adoption of iFR as a single measure in clinical decision making. The DEFINE-FLAIR trial (NCT 02053038) will compare strategies of revascularization guided by iFR and FFR in 2,500 patients requiring physiological interrogation in clinical practice. The primary outcome will be the rate of major adverse cardiac events (composite of death, myocardial infarction, and unplanned revascularization) at 1 year (Figure 7). Other studies, such as the SYNTAX II, Prospect II, and iFR-SWEDEHEART will further evaluate the merits of iFR in clinical decision making.
Figure 7.
The FLAIR trial will evaluate the clinical merits of iFR guided revascularization.
Limitations
The present study only performed a direct comparison between the classification of stenoses by iFR and FFR. No alternative noninvasive method was applied in this analysis, which prevents a more in-depth interpretation of iFR-FFR disagreements.
There was no active recruitment of study subjects. Patients included in this study represent the selection of clinicians based on a clinical indication for FFR measurement. Also, no formal measurement protocol for iFR or FFR was suggested, and technical aspects were left to the discretion of the interventionalist according to their routine clinical practice. Also, iFR and FFR measurements were not blinded from each other, as the clinical operator had access to both results.
Although each individual FFR value might have been different if different doses or routes of adenosine were used, it is likely that on average, across the whole study population, this would not affect the overall relationship with iFR because this variability would likely occur in both ways (more or less hyperemia). Importantly, as a retrospective analysis of the routine clinical application of FFR, our study used no fixed protocol of adenosine administration, which means that our results entirely reflect the choice of clinicians and the way FFR is used everyday around the world. This can be seen as a strength of our study because the relationship between iFR and FFR, therefore, reflects the clinical relationship expected when iFR is used for clinical reasons. Also, the recently presented ADVISE II study,24 which used a rigid protocol of central IV adenosine administration, demonstrated a level of agreement between iFR and FFR equal to our study, which again confirms the validity of our findings.
Finally, the present study cannot evaluate the impact of iFR use on clinical outcomes because we simply described its diagnostic agreement with FFR in clinical practice. This merit will be addressed by the DEFINE-FLAIR study (Figure 7) and other future trials.
Conclusion
Real-time iFR measurement in the cardiac catheterization laboratory is feasible and accessible to clinicians, delivering the same magnitude of agreement between iFR and FFR as described in core laboratory studies. Outcome studies are needed to evaluate the clinical value of iFR as an independent decision-making tool in such population.
Disclosures
J.E. Davies holds patents pertaining to iFR technology, which is under licence to Volcano Corporation. J.E. Davies is a consultant for Volcano Corporation. The other authors have no conflicts of interest to declare.
References
- 1.Sen S., Escaned J., Malik I.S. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59(15):1392–1402. doi: 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Petraco R., Escaned J., Sen S. Classification performance of instantaneous wave–free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. Eurointervention. 2013;9(1):91–101. doi: 10.4244/EIJV9I1A14. [DOI] [PubMed] [Google Scholar]
- 3.Park J.J., Petraco R., Nam C.W. Clinical validation of the resting pressure parameters in the assessment of functionally significant coronary stenosis; results of an independent, blinded comparison with fractional flow reserve. Int J Cardiol. 2013;168(4):4070–4075. doi: 10.1016/j.ijcard.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Jeremias A., Maehara A., Genereux P. Multicenter core laboratory comparison of the instantaneous wave–free ratio and resting P/P with fractional flow reserve: the RESOLVE study. J Am Coll Cardiol. 2013;63(13):1253–1261. doi: 10.1016/j.jacc.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 5.Sen S., Asrress K.N., Nijjer S. Diagnostic classification of the instantaneous wave–free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration: results of CLARIFY (Classification Accuracy of Pressure-Only Ratios Against Indices Using Flow Study) J Am Coll Cardiol. 2013;61(13):1409–1420. doi: 10.1016/j.jacc.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Sen S, Nijjer S, Petraco R, Malik IS, Francis DP, Davies J. Letter to the editor: instantaneous wave–free (iFR): numerically different, but diagnostically superior to FFR? Is lower always better? J Am Coll Cardiol. 2013;62(6):566. doi: 10.1016/j.jacc.2013.03.076. [DOI] [PubMed] [Google Scholar]
- 7.van de Hoef T.P., Meuwissen M., Escaned J. Head-to-head comparison of basal stenosis resistance index, instantaneous wave-free ratio, and fractional flow reserve: diagnostic accuracy for stenosis-specific myocardial ischaemia. EuroIntervention. 2014 doi: 10.4244/EIJY14M08_17. (pii: 20130905-03) [DOI] [PubMed] [Google Scholar]
- 8.Jeremias A. TCT 2012, Oral Presentation. 2012. RESOLVE: a multicenter study evaluating the diagnostic accuracy of iFR compared to FFR. [Google Scholar]
- 9.Petraco R., Park J.J., Sen S. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularisation. Eurointervention. 2013;8(10):1157–1165. doi: 10.4244/EIJV8I10A179. [DOI] [PubMed] [Google Scholar]
- 10.Kern M.J., Lerman A., Bech J.W. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114(12):1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 11.van de Hoef T.P., Meuwissen M., Escaned J. Fractional flow reserve as a surrogate for inducible myocardial ischaemia. Nat Rev Cardiol. 2013;10(8):439–452. doi: 10.1038/nrcardio.2013.86. [DOI] [PubMed] [Google Scholar]
- 12.Bech G.J.W., De Bruyne B., Pijls N.H.J. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–2934. doi: 10.1161/01.cir.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 13.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 14.Wijns W., Kolh P., Danchin N. Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31(20):2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 15.Tonino P.A.L., De Bruyne B., Pijls N.H.J. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyne B., Pijls N.H.J., Kalesan B. Fractional flow reserve–guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 17.Pijls N.H.J., de Bruyne B., Peels K. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–1708. doi: 10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 18.Pijls N.H.J., van Schaardenburgh P., Manoharan G. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER study. J Am Coll Cardiol. 2007;49(21):2105–2111. doi: 10.1016/j.jacc.2007.01.087. [j.jacc.2007.01.087] [DOI] [PubMed] [Google Scholar]
- 19.Li J., Elrashidi M.Y., Flammer A.J. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34(18):1375–1383. doi: 10.1093/eurheartj/eht005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petraco R., Sen S., Nijjer S. Fractional flow reserve-guided revascularization: practical implications of a diagnostic gray zone and measurement variability on clinical decisions. J Am Coll Cardiol Intv. 2013;6(3):222–225. doi: 10.1016/j.jcin.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Petraco da Cunha R., van de Hoef T., Nijjer S. The performance of baseline haemodynamic indices of coronary disease severity in the DEFER and FAME studies: an estimated agreement of stenoses classification with fractional flow reserve. J Am Coll Cardiol. 2013;61 [10_S] [Google Scholar]
- 22.Kleiman N.S. Bringing it all together: integration of physiology with anatomy during cardiac catheterization. J Am Coll Cardiol. 2011;58(12):1219–1221. doi: 10.1016/j.jacc.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Akdeniz C., Umman S., Nisanci Y. Percutaneous coronary intervention increases microvascular resistance in patients with non–ST-elevation acute coronary syndrome. Eurointervention. 2013;9(2):228–234. doi: 10.4244/EIJV9I2A38. [DOI] [PubMed] [Google Scholar]
- 24.Escaned J. TCT 2013, Oral Presentation. 2013. ADenosine Vasodilator Independent Stenosis Evaluation II (ADVISE II) [ NCT01740895] [Google Scholar]