Abstract
Aim:
Metabolic syndrome (MS) and aging are low-grade systemic inflammatory conditions, and inflammation is a key component of endothelial dysfunction. The aim of this study was to investigate the effects of non-steroidal anti-inflammatory drugs (NSAIDs) upon the vascular reactivity in aging MS rats.
Methods:
MS was induced in young male rats by adding 30% sucrose in drinking water over 6, 12, and 18 months. When the treatment was finished, the blood samples were collected, and aortas were dissected out. The expression of COX isoenzymes and PLA2 in the aortas was analyzed using Western blot analysis. The contractile responses of aortic rings to norepinephrine (1 μmol/L) were measured in the presence or absence of different NSAIDs (10 μmol/L for each).
Results:
Serum levels of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) in control rats were remained stable during the aging process, whereas serum IL-6 in MS rats were significantly increased at 12 and 18 months. The levels of COX isoenzyme and PLA2 in aortas from control rats increased with the aging, whereas those in aortas from MS rats were irregularly increased with the highest levels at 6 months. Pretreatment with acetylsalicylic acid (a COX-1 preferential inhibitor), indomethacin (a non-selective COX inhibitor) or meloxicam (a COX-2 preferential inhibitor) decreased NE-induced contractions of aortic rings from MS rats at all the ages, with meloxicam being the most potent. Acetylsalicylic acid also significantly reduced the maximum responses of ACh-induced vasorelaxation of aortic rings from MS rats, but indomethacin and meloxicam had no effect.
Conclusion:
NSAIDs can directly affect vascular responses in aging MS rats. Understanding the effects of NSAIDs on blood vessels may improve the treatment of cardiovascular diseases and MS in the elders.
Keywords: NSAID, metabolic syndrome, aging, cytokine, cyclooxygenase, aortic ring, vascular reactivity, norepinephrine, acetylcholine
Introduction
Metabolic syndrome (MS) and aging are low-grade systemic inflammatory conditions, and inflammation is a key component of endothelial dysfunction1. Inflammation in MS is mediated by secretory agents from adipose tissue and the liver. These agents alter the function of different tissues and organs and contribute to cardiovascular complications. MS and type-1 and type-2 diabetes cause an imbalance between the production of vasodilating and vasoconstricting prostanoids in arteries from rodent models that underlies endothelial dysfunction and abnormal vascular smooth muscle reactivity2,3,4. Anti-inflammatory drugs, particularly non-steroidal, are beneficial for patients with vascular diseases1. MS accelerates the aging process, and many predisposing conditions that increase in prevalence during aging, such as obesity, insulin resistance (IR), inflammation, stress and hypertension, also contribute to an increased prevalence of MS5.
The endothelial dysfunction caused by inflammation in MS and aging could be explained by the withdrawal of endothelial inhibitory signals, such as prostacyclin, nitric oxide (NO), and endothelium-derived hyperpolarizing factor (EDHF), or the production of vasoconstricting substances. Endothelial-dependent relaxation (EDR) decreases with age in the large vessels of different animal species, including humans. Impaired ACh-induced EDR in aged rat aortas is partly due to a decrease in basal NO release, endothelial NO synthase (eNOS) expression and phosphorylation-mediated eNOS activation. However, during aging, the local formation of reactive oxygen and nitrogen species and endothelium-derived contracting factors (EDCF), such as angiotensin II, endothelin-1 and vasoconstricting prostanoids are increased6.
The mechanism of the endothelium-derived hyperpolarization (EDH) involves an increase in endothelial [Ca2+]i and activation of localized small and/or intermediate conductance calcium-activated potassium channels (SKCa and SK3). The subsequent endothelial hyperpolarizing current is then transferred to the smooth muscle via myoendothelial gap junctions (MEGJs), and endothelial K+ is released, which activates smooth muscle Na/K+-ATPase, closing the smooth muscle voltage-dependent calcium channels, thereby hyperpolarizing the smooth muscle and dilating the artery7. The contribution of KCa subtypes and MEGJs to EDH varies during aging8.
Studies in humans9 and rats10 suggest that treatment with low-dose aspirin is able to reverse EDR dysfunction. Some studies have suggested that the release or effect of cyclooxygenase (COX)-dependent vasoactive factors may also contribute to endothelial dysfunction in aging11.
Non-steroidal anti-inflammatory agents (NSAIDs) constitute the group of agents most employed for effective protection against pain and inflammation12. Their action is mainly due to blocking prostaglandin synthesis by inhibiting COX, which converts arachidonic acid into cyclic endoperoxides, which are precursors of prostaglandins13. NSAIDs have different effects depending upon the dose used and the cell type affected. Furthermore, a high prevalence of diseases, such as hypertension, diabetes, atherosclerosis, osteoarthritis and cancer, in elderly patients promotes an increased use of NSAIDs14.
Reports on the effect of NSAIDs on the cardiovascular system are controversial15,16,17,18. NSAIDs cause increased blood pressure by blocking the synthesis of prostaglandins that regulate vascular tone and sodium excretion19,20. Low-doses of aspirin and selective COX-2 inhibitors can either improve or worsen endothelial dysfunction in hypercholesterolemia, atherosclerosis and hypertension according to different authors18,21.
There are two isoforms of cyclooxygenases, known as COX-1 and COX-2. COXs participate in numerous physiological functions and pathological disorders associated with endothelial dysfunction22. COX-1, a known target of low-dose aspirin, is constitutively expressed in most tissues to regulate the synthesis of prostaglandins. Although COX-2 is induced as part of the inflammatory response, COX-2 has recently been reported to be constitutively expressed in the vascular endothelium20,23,24,25.
COX-2 is increased in blood vessels of individuals with cardiovascular risk factors26. Recently, the prostanoid production from constitutively expressed COX-2 has been shown to be involved in modulating vascular responses27,28,29. In animal models, selective inhibition of COX-2 promotes hypertension, atherogenesis, and the formation of thrombi, which are all risk factors for acute myocardial infarction. Nevertheless, the exact pathogenesis of the increased rate of cardiovascular complications caused by coxibs is unclear at this point30.
We have studied changes in blood pressure and vascular contractility in a rat model of MS, caused by chronic ingestion of sucrose, developed at our Institution, showing that with aging there is endothelial dysfunction. The sucrose fed rat develops central obesity, moderate hypertension, hypertriglyceridemia and hyperinsulinemia31.
Therefore, MS and aging are inter-related conditions in which there is systemic inflammation that induces endothelial dysfunction. The role of NSAIDs in modifying COX-1 and/or COX-2 activity in blood vessels and thereby preventing endothelial dysfunction in these conditions is controversial. Thus, the purpose of the present work was to determine the effect of NSAIDs (acetyl-salicylic acid, indomethacin and meloxicam) on vascular reactivity in isolated aortas from mature (6 months old, when MS starts) and aged (12 and 18 months old) rats. Understanding the effect of NSAIDs on blood vessels could help improve the treatment of cardiovascular diseases and MS in older people.
Materials and methods
Animals
The experiments in animals were approved by the Laboratory Animal Care Committee of our Institution and were conducted in compliance with our Institution's Ethical Guidelines for Animal Research.
Weanling male Wistar rats aged 25 d and weighing 50±4 g were separated into two groups: group 1, Control rats (Control), which were given tap water to drink; and group 2, MS rats, which were given 30% sucrose in drinking water over 6, 12, and 18 months. At least 8 animals were used per group.
All animals were fed Purina 5001 rat chow (Richmond, IN, USA) ad libitum, which provides 14.63 KJ/g, with 23% protein, 12% fat and 65% carbohydrate, under controlled temperature and a 12:12-h light/dark cycle.
Systolic arterial blood pressure was measured in conscious animals using the tail cuff method; the cuff was connected to a pneumatic pulse transducer (Narco Bio-systems Inc, Healthdyne Co, Austin, TX, USA) and a programmed electrosphyngomanometer. The mean of seven independent determinations was calculated.
Blood sample collection and determination of glucose, insulin, leptin, adiponectin, triglycerides, and pro-inflammatory cytokines
After overnight fasting (12 h), the animals were killed by decapitation, and blood was collected. The serum was separated by centrifugation at 600×g for 15 min at room temperature and stored at −70 °C until needed. Serum insulin, adiponectin and leptin were determined using commercial radioimmunoassay (RIA) kits specific for rats (Linco Research Inc, St Charles, MO, USA); the sensitivity was 0.1 ng/mL and intra- and inter-assay coefficients of variation were 5%, 10%, and 10%, respectively. Glucose concentration was assayed using the enzymatic kit SERA-PAK Plus (Bayer Corporation, Sées, France). Triglycerides were measured using commercially available procedures (Randox, Laboratories LTD, Antrim, UK). The cytokines interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) were quantified by ELISA (PeproTech, Jersey City, NJ, USA).
Sample preparation and vascular reactivity
The animals were killed by decapitation, and the aortas were immediately dissected and placed in oxygenated normal Tyrode solution (mmol/L: 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, and 5.5 glucose; pH 7.4). The arteries were carefully cleaned from connective and adipose tissue, taking care not to damage the endothelium. Tension measurements were made as previously described31. A 2 g basal passive tension was applied to aortic rings from the Control and MS animals. This tension has been tested previously and found to be optimal under our experimental conditions31. The arteries were allowed to rest for 60 min, with replacement of the Tyrode solution every 20 min. The arteries were stimulated twice with norepinephrine (NE, 1 μmol/L), and the mean values obtained were considered to be 100% of the contractile responses. To test the integrity of the endothelium, NE (1 μmol/L)-precontracted arteries were challenged with 10 μmol/L acetylcholine (ACh). The arteries that did not develop ACh-induced vasorelaxation were discarded. The vasodilator activity was determined by cumulative concentration-response curves to ACh (0.1 nmol/L to 1 μmol/L) on NE (1 μmol/L)-precontracted aortic rings.
To assess the participation of COX metabolites in mediating the vascular responses to NE and ACh, the curves were repeated in the presence of NSAIDs. The preparations were exposed for 30 min to 10 μmol/L of acetylsalicylic acid (ASA, a COX-1 preferential inhibitor), indomethacin (a non-selective COX inhibitor) or meloxicam (a COX-2 preferential inhibitor).
PLA2, COX-1, and COX-2 expression
Protein expression was examined by Western blot analysis. Frozen thoracic aortic samples were homogenized (25% w/v) in a lysis buffer (pH 7.4), containing 250 mmol/L sucrose, 10 mmol/L Tris, 1 mmol/L EDTA,1 mmol/L phenyl-methylsulfonyl fluoride (PMSF), 2 μmol/L pepstatin A, 2 μmol/L leupeptin, and 0.1% aprotinin, at 4 °C. The homogenate was centrifuged at 900×g for 10 min at 4 °C. The supernatant was separated and kept at −70 °C until required. The protein concentration was determined by the method of Lowry et al32.
A total of 100 mg protein was separated by SDS-PAGE (8% polyacrylamide gel) and transferred to a nitrocellulose Hybond-P membrane (Millipore). The blots were blocked for 3 h at room temperature with Tris buffer solution (TBS), containing 5% nonfat dry milk and 0.05% Tween 20. The membranes were incubated overnight at 4 °C with rabbit primary polyclonal antibodies against phosoholipase A2 (PLA2), COX-1 and COX-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a final dilution of 1:1000. The blots were washed in TBS and incubated for 3 h at room temperature with a 1:1000 dilution of goat secondary polyclonal antibodies (anti-rabbit peroxidase-labeled, Santa Cruz Biotechnology). After incubation with the secondary antibody, the membranes were washed with TBS, and band detection was performed using 3,3'-diaminobenzidine.
After identifying the relevant protein, the membranes were stripped by washing with a buffer of 1% Tris, 1% SDS, and 100 mmol/L β-mercaptoethanol (pH 2) for 2 h, followed by a washing with TBS. The membranes were blocked for 3 h and incubated overnight with a mouse monoclonal biotinylated α-actin antibody (1:2000) as a protein loading control. The membranes were analyzed by densitometry using 1D image analysis software, Windows Version 3.5. The density values for each band are expressed as optical density units.
Drugs and reagents
The drugs and all other reagents were purchased from Sigma Chemical Co.
Statistical analysis
The results are expressed as the mean±standard errors of the mean (SEM) from 6 to 10 different artery preparations. The percentage of contraction in each experiment was calculated, and the mean was determined. When applicable (comparisons between two values; Control and MS), statistical analysis was performed using Student's t test. Comparisons among groups were performed by two-way analysis of variance (ANOVA), using the Sigma Stat program (Jandel Scientific). The IC50 and maximum dilation response (Emax) values from the concentration–response curves of ACh for relaxation of the rat aorta were performed using the Sigma Plot (Systat Software, San Jose, CA, USA) program. Differences were considered statistically significant when P<0.05.
Results
Changes in body weight, abdominal fat, arterial pressure, triglycerides, glucose, insulin, leptin, and adiponectin
Table 1 summarizes the characteristics for the groups of rats used. At six months, the experimental animals developed MS characterized by hypertension, hypertriglyceridemia, hyperinsulinemia and IR. There was not a statistically significant difference in weight between the Control and MS rats; however, the MS animals showed an accumulation of abdominal fat. Body weight, abdominal fat, triglycerides and leptin concentrations were significantly increased in the 18-month-old Control rats. In the 18-month-old MS rats, weight, visceral fat and triglycerides were higher than the young MS rats. Serum triglycerides and leptin were significantly higher in the MS rats than the Control rats, and the levels increased with age. At six months of age, the MS rats had higher adiponectin levels than the Control rats. The adiponectin concentration did not change significantly during aging in the Control animals but increased with age in the MS animals.
Table 1. Characteristics and biochemical parameters from Control and MS rats during aging. Values are mean±SEM. n=8. cP<0.01 vs Control at corresponding age. eP<0.05 vs 6 and 12 months of age in the same group, hP<0.05 vs 6 months of age in the same group.
| Control |
MS |
|||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | 6 months | 12 months | 18 months | |
| Body weight (g) | 516.2±8.5 | 573.3±19.6 | 616.3±12.8e | 526.2±14.3 | 649.3±50.7 | 817.3±30.9ce |
| Central adiposity (g) | 5.9±0.7 | 9.2±0.5 | 13.1±1.6e | 11.9±0.6c | 18.7±1.7c | 40.6±3.9ce |
| Blood pressure (mmHg) | 103.4±0.6 | 103.2±1.2 | 100.1±0.7 | 143.2±0.7c | 109.9±5.9c | 95.9±1.1ce |
| Glucose (mmol/L) | 5.9±0.3 | 5.2±0.2 | 5.6±0.4 | 4.8±0.7 | 6.1±0.4 | 6.3±0.2 |
| Triglycerides (mg/dL) | 55.6±4.7 | 93.2±6.3h | 155.8±8.9e | 109.1±12.8c | 200.4±30.3ch | 266.2±28.9ch |
| Insulin (μU/mL) | 6.5±0.9 | 6.5±2.4 | 6.4±0.7 | 18.2±3.5c | 17.4±6.7 | 7.5±1.6h |
| Leptin (ng/mL) | 1.0±0.2 | 3.5±0.6 | 3.5±0.5h | 3.9±0.5c | 10.8±1.9c | 20.2±2.6ce |
| Adiponectin (μg/mL) | 3.1±0.2 | 3.5±0.3 | 3.2±0.2 | 5.5±0.4c | 7.1±0.7c | 7.8±0.4ch |
The fasting serum glucose levels were not significantly different among the groups, but there was an increase at 18 months in the Control and MS rats. Although there was a tendency for an increased insulin level at all ages, serum insulin was only significantly increased at 6 months in the MS rats compared to the Control. In the MS rats, the insulin level significantly decreased from 12 to 18 months.
In the Control rats, the arterial blood pressure showed no significant variation during aging. Systolic arterial pressure was significantly elevated in the MS rats compared to the Control rats at 6 and 12 months of age, showing a maximum at 6 months (143.2±0.7 mmHg) before decreasing toward the end of treatment, reaching a value even lower than the Control animals at 18 months (95.9±1.1 mmHg).
Cytokine levels
The serum concentration of IL-6 was not significantly different in the Control and MS rats at 6 months of age; nevertheless, as the Control rats aged, there was no variation in its concentration, while in the MS rats, IL-6 increased at 12 and 18 months of age (Table 2). IL-1β levels remained constant during aging in the Control rats and decreased during aging in the MS group. There are no significant differences between groups for TNF-α level, which remained unchanged during aging (Table 2).
Table 2. Serum concentrations of pro-inflammatory cytokines of both Control and MS rats during aging. Values are mean±SEM. n=8. cP<0.01 vs Control at corresponding age. hP<0.05 vs younger animals of age in the same group.
| Cytokine (pg/mL) | Control |
MS |
||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | 18 months | 6 months | 12 months | 18 months | |
| IL-1β | 135.6±15.6 | 93.5±13.2 | 164.9±10.2 | 149.7±9.4 | 95.1±9.0 | 88.0±9.3ch |
| TNF-α | 253.5±37.4 | 290.2±20.0 | 327.5±49.1 | 388.8±47.1 | 275.0±40.1 | 320.1±20.6 |
| IL-6 | 436.8±70.5 | 391.1±23.5 | 496.6±29.2 | 432.8±20.8 | 586.9±39.9ch | 798.1±55.3ch |
Western-blot analysis of the COXs and PLA2
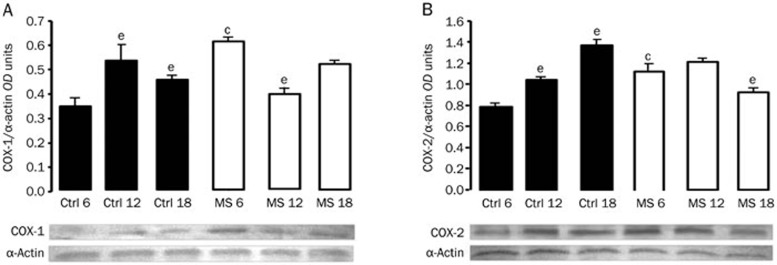
To address the effect of aging on the expression of the enzymes that participate in arachidonic acid metabolism in the aorta, we performed immunoblotting analyses. Figure 1 show (70 KDa) COX-1 and COX-2 expression, respectively, for the Control and MS rats during aging. At 6 months of age, expression of the aortic COX isoforms in the MS rats increased compared to the Control rats. When assessing the effect of aging, we observed an increase in the expression of both isoenzymes in the Control group, while in the MS animals, there was a tendency toward a decrease.
Figure 1.
Representative Western blots for (A) COX-1 and (B) COX-2. Protein expression of the enzyme isoforms was evaluated in thoracic aortas from Controls and MS rats during aging. The bars represent the mean±SEM of 8 animals per group. cP<0.01 vs Control at corresponding age. eP<0.05 vs 6 months of age in the same group.
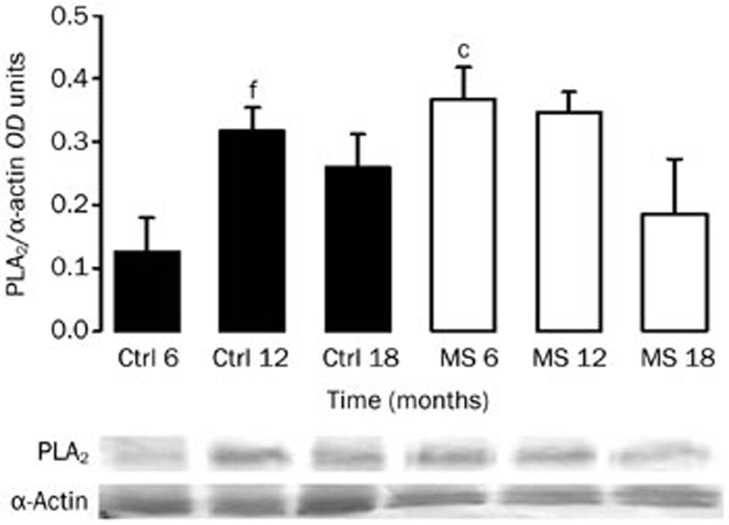
The 85 kDa PLA2 isoform was expressed in the aortas from adult rats (Figure 2). Similar to COX isoform expression, PLA2 was significantly increased in the aortas from MS rats at 6 months of age. The expression was increased in arteries from the old Control rats. However, in the MS rats, the expression of this enzyme showed no significant variation during aging.
Figure 2.
Representative Western-blot for PLA2. Protein expression of the enzyme was evaluated in aortas from Controls and MS rats during aging. The bars represent the mean±SEM of 8 animals per group. cP<0.01 vs Control at corresponding age. fP<0.01 vs 6 months of age in the same group.
Contraction to NE and relaxation with ACh in the Control and MS rats during aging
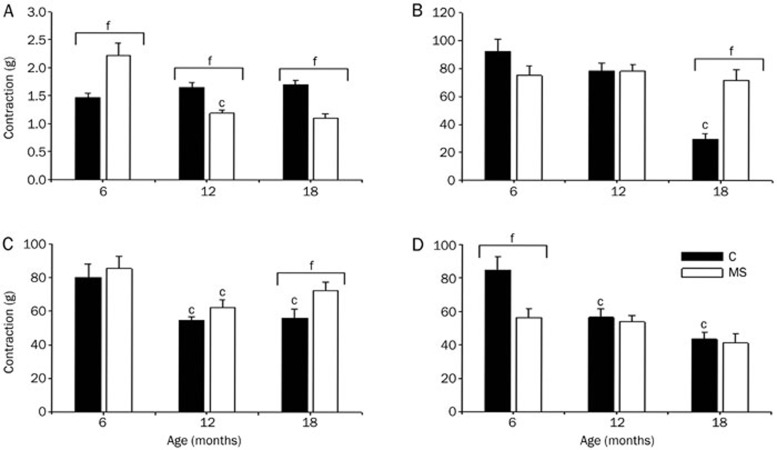
At 6 months of age, NE-induced aortic contraction was stronger in the vessels from MS rats than Control vessels. NE-induced vascular contraction was not modified during the aging period studied in the Control rats; however, this contraction decreased with age in the aortas from MS rats (Figure 3A).
Figure 3.
Vascular contractile responses to NE (1 μmol/L) in the Control (solid bars) and MS (open bars) rats during aging. (A) Without NSAIDs. The data are normalized using the control contraction at each age as 100% (panels B, Control and D); 100% contraction corresponds to tension in grams as shown in panel A. (B) Pretreatment of the aortic rings for 30 min with a single dose of ASA (10 μmol/L). (C) Indomethacin and (D) meloxicam. The data are the mean±SEM of at least 6 measurements. cP<0.01. fP<0.01 vs 6 months of age in the same group.
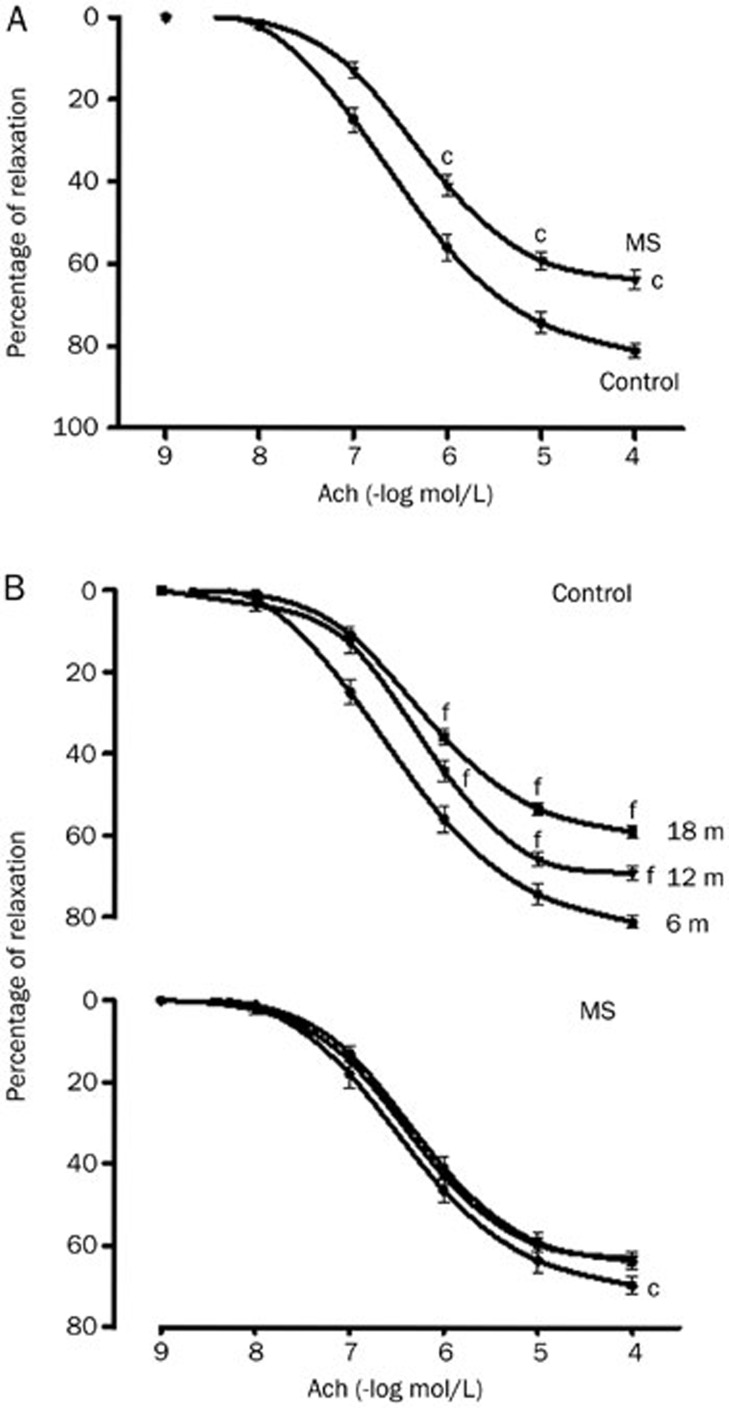
The ACh relaxation in NE-precontracted rat aortic rings was concentration-dependent. Premature endothelial dysfunction was observed in rats with MS (6 months old) (Figure 4A); the relaxing capacity of the aortas gradually diminished with age in the Control group, while in the MS group, the aortas already had a level of relaxation compared to the aged Control and remained at this level during aging (Figure 4B). The dilatory dose-response curves of the aorta to ACh indicated that the endothelium-dependent relaxation was impaired in the MS rats and old Control rats (maximal relaxation of 63.0%±1.8% and 59.0%±1.6%, respectively, compared to 81.0%±1.5% in the Control rats at 6 months). The sensitivity to ACh, as reflected by the EC50, was not altered in the MS group; whereas in the older Control rats, the sensitivity was significantly lower compared to the young rats (Figure 4C and Table 3).
Figure 4.
ACh-induced vasorelaxation in NE-precontracted aortic rings from 6-month-old Control and MS rats (A) and during aging in both groups (B). The data are mean±SEM of at least 6 measurements. cP<0.01 MS vs Control rats at 6 months of age. fP<0.01 for Controls rats at 12 and 18 months of age vs Controls rats at 6 months of age.
Table 3. Effect of ASA on EC50 and maximum dilation (Emax) values of Ach-induced relaxation of aortas of 6, 12, 18 month-old Control, and MS rats.
| Age (months) | Without ASA |
ASA |
|||
|---|---|---|---|---|---|
| EC50 (mol/L) | Emax (%) | EC50 (mol/L) | Emax (%) | ||
| Controls | 6 | 3.2×10−7±6.4×10−8 | 81.0±1.5 | 1.7×10−6±7.4×10−7c | 56.8±4.8c |
| 12 | 8.7×10−7±2.3×10−7 | 69.1±1.6 | 7.2×10−7±1.1×10−7 | 66.1±2.5 | |
| 18 | 1.4×10−6±4.2×10−7e | 59.0±1.6e | 1.1×10−6±3.8×10−7 | 57.9±1.3 | |
| MS | 6 | 4.1×10−7±7.3×10−8 | 63.7±2.2 | 4.3×10−7±5.0×10−8 | 64.9±1.7 |
| 12 | 4.1×10−7±9.4×10−8 | 69.6±2.2 | 4.2×10−7±6.7×10−8 | 66.7±3.4 | |
| 18 | 4.9×10−7±9.5×10−8 | 63.0±1.8 | 6.6×10−7±1.8×10−7 | 51.5±6.2c | |
Aortic rings were pre-constricted with NE 1 μmol/L. Changes in the maximum response (Emax, expressed as a percentage of relaxation) and EC50 to Ach in aortas from Control and MS rats. Values are mean±SEM. n=8. eP<0.05 vs other ages in the same group. cP<0.05 vs without treatment.
Effect of NSAIDs on vascular contraction
Throughout aging, ASA gradually reduced the contraction elicited by NE in aortic rings from Control rats (8% at 6, 22% at 12, and 70% at 18 months old). Indomethacin significantly diminished vasoconstriction more in the Control old rats than Control young rats. At 6 months of age, NE-contraction was significantly lower in the meloxicam-treated aortic rings from MS rats than Control aortas. NSAIDs decreased vascular contraction in the same proportion in all ages studied in the MS rats, while meloxicam was the most potent (Figure 3B–3D).
Effect of NSAIDs on ACh-induced vasorelaxation
To evaluate the activity of each COX in controlling vascular tone, a second dose–response curve to ACh was obtained with or without COX-1 and COX-2-selective inhibitors. In the aortas from young Control rats, endothelium-dependent relaxation was significantly diminished by ASA compared to the response in old rats (Table 3). In contrast, ASA significantly reduced the maximum response to ACh without changing sensitivity (ie, potency) in the aortas from old MS rats (Table 3). Indomethacin and meloxicam showed no effect on vasodilation in the aortas from Control and MS rats at any age studied (data not shown).
Discussion
Inflammation is one of the main mechanisms underlying endothelial dysfunction and therefore plays an important role in atherosclerosis and other cardiovascular diseases, such as hypertension, IR, dyslipidemias and obesity, which are hallmarks of MS1.
During aging, the development of IR and cardiovascular diseases are accelerated by MS33,34. Obesity and aging are two overlapping and mounting public health problems in which low grade systemic inflammation is a common underlying condition. The prevalence of obesity is related to the increasing prevalence of MS, which is growing progressively even among older age groups. Aging is also associated with immunological changes (immunosenescence) that resemble those observed following chronic stress or glucocorticoid treatment. Immunosenescence is related to changes in peripheral glucocorticoid levels35.
In this work, we determined the effect of NSAIDs upon vascular reactivity in isolated aortas from mature (6 months old, when MS starts) and aged (12 and 18 months old) Control and MS rats. We measured the serum levels of various variables to prove the presence of MS. Triglycerides were increased at all ages in our experimental MS group. Glucose was increased in the MS and Control rats at 18 months and is therefore a consequence of aging. Impaired glucose metabolism with age represents a major determinant of the epidemic of type 2 diabetes within the elderly population36. Insulin was increased at 6 months, and IR was present (indicated by HOMA-IR) in the MS rats. This increase was accompanied by the maximal blood pressure and NE-induced contractility found in this paper. Values for all of these variables decreased after this age. In the MS rats, the increase in glucose could be due to the significantly reduced insulin levels found in the old animals, which could be a consequence of age and the experimental treatment. This result is consistent with experimental data from different species showing that aging per se is associated with a continuous decrease in basal insulin release. The magnitude of this effect is enough to develop abnormalities in glucose metabolism36,37,38.
Body weight increased in the Control and MS rats; nevertheless, the difference between the groups was not significant even though the diet of the sucrose-fed rats was hypercaloric (Table 1). The sucrose-fed animals showed increased central adiposity, which is one of the characteristics of MS animals. The increase in abdominal fat was most likely accompanied by a decrease in muscle mass as reported by other groups39 because body weight did not significantly increase. In our model, we have not determined a difference in muscle mass between the Control and MS rats, but sucrose fed animals have been shown to consume less solid food, which means less protein and mineral intake40. Although obesity is a risk factor for sarcopenia, its pathophysiology is complex, and multiple factors, including lifestyle, endocrine, and immunological factors, can play a role. Moreover, aging is associated with important changes in body composition and metabolism, and there are reports of the presence of sarcopenia and centralized fat in the elderly41,42.
Obesity contributes to inflammation in MS and diabetes. The increase in adipose tissue mass induces a state of systemic inflammation due to an increase in secretory factors derived from pre-adipocytes (adipokines) and macrophages constituting this tissue. This inflammation significantly contributes to the endothelial dysfunction present in cardiovascular diseases43,44. Leptin and adiponectin were elevated in MS, and both adipokines increased with age in the Control and MS rats in our experiments.
Adiponectin is a newly described anti-inflammatory protein secreted exclusively by adipocytes and plays a protective role against IR and endothelial vascular function. Age-related changes in adiponectin levels remain controversial45. In older populations, a higher adiponectin concentration was associated with a greater risk of cardiovascular disease, stroke and mortality. However, other authors have found no association between adiponectin and the risk of stroke46.
Leptin is an adipokine that is now considered to control lipoprotein function, acute phase reactants, glucocorticoid metabolism, inflammation, immune function and reproduction and, hence, is key to integrating adipose tissue with competing biological functions47. Leptin also increases reactive oxygen species in endothelial cells and stimulates the secretion of pro-inflammatory cytokines48. Therefore, the high concentration of leptin found in this paper in MS rats and older animals may be regarded as a marker of inflammation (Table 1). MS is strongly linked to an increase in systemic inflammation markers, such as C-reactive protein, IL-6 and TNF-α33,34.
Aging per se, in the absence of other risk factors (ie, MS), is associated with oxidative stress and inflammatory changes in blood vessels. Arterial endothelial and smooth muscle cells produce and secrete TNF-α and contribute to its elevated plasma concentration in older organisms. Adipocytes are another significant source of circulating TNF-α. Some authors have linked TNF-α to endothelial impairment during aging. The effects induced by TNF-α closely mimic aging-induced functional and phenotypic alterations in the arterial endothelium, such as the induction of NO synthase, COX-2 and sPLA2 in various cell types49,50. Likewise, there are several reports that define aging as a chronic inflammatory process (an imbalance between pro- and anti-inflammatory activity). Additionally, high levels of a wide variety pro-inflammatory cytokines and markers, such as IL-1β, IL-6, fibrinogen and adhesion molecules, have been found in the serum of elderly patients51.
Our results show that serum pro-inflammatory cytokine levels remained stable during aging in the Control rats, even in the presence of a high amount of visceral fat. However, in the MS group, IL-6 expression increased at 12 and 18 months. Contrary to the change in IL-6, serum IL-1β decreased in the 18-month-old MS rats (Table 2). This decrease may be due, in part, to the systemic anti-inflammatory effect exerted by adiponectin, which increased in the serum of old MS animals (Table 1).
Further research is needed to identify signs of local inflammation in the vessels, but COX-2 and PLA2 overexpression in the aorta may be indicative of the inflammation present in MS and aging rats. Moreover, prostaglandin formation by COX-2 and NO formed by iNOS are two predominant small-molecule mediators of inflammation. COX-2 and iNOS seem to work synergistically52.
Although the etiology of vascular disorders in MS and aging is not completely studied, alterations in vascular reactivity to neurotransmitters and hormones might be responsible for the abnormal functioning of blood vessels. In Control rats, NE-induced vascular contraction was not modified during aging. In contrast, in the aortas from MS rats, contraction was higher compared to the Control at 6 months and then decreased with age (Figure 3A). We had previously studied aortic contractility to KCl and found that contraction to KCl was not modified during aging in the Control rats but increased at 4 and 6 months in the MS rats and decreased thereafter, similar to what we found with NE in this paper31.
Endothelium-dependent contraction involves the production of reactive oxygen species and COX-1 activation. At least, in the rat aorta, EDCFs appear to be COX-1-derived prostanoids generated in the endothelium, which diffuse to contract the underlying vascular smooth muscle by activating thromboxane–prostanoid receptors53. Therefore, EDCF diffuses and subsequently stimulates thromboxane-prostanoid receptors in vascular smooth muscle54. The involvement of COX and prostanoid production depends on the vascular bed and the body's condition. In diseases, such as hypertension, diabetes and MS, there is an imbalance in the production and release of prostanoids.
Some effects of NSAIDs on the vasculature have been reported, but the mechanisms responsible for these effects are not fully understood26. In the older human population, people frequently have multiple problems. A large number of people receiving drug treatment for hypertension have arthritis, which requires medication for pain relief. Most of the agents used for pain relief inhibit COX.
The effects of NSAIDs have been investigated in people with and without elevated blood pressure, and the effects were reviewed in a meta-analysis in 1994. An important question is whether there are differences between the various NSAIDs55. The mechanism by which blood pressure rises with NSAIDs is not certain. Most likely, the main mechanism is inhibition of prostaglandin synthesis because NSAIDs have a higher propensity to increase blood pressure, in which regulation (and renal function) is more prostaglandin-dependent. NSAIDs also interact with drugs (diuretics, beta-blockers and ACE inhibitors) that may exert effects through increased prostaglandin formation. In contrast, NSAIDs do not interact with calcium antagonists and central acting drugs, which have actions that are apparently unrelated to renal/extrarenal production of prostaglandins. Inhibition of natriuretic prostaglandins could explain the pressure effects of NSAIDs in treated hypertensive patients, but sodium retention may not be the single explanation for such an interaction56.
NSAIDs, particularly the 'coxibs', have risky cardiovascular side effects that might be related to the tendency of some of these drugs to elevate blood pressure, and the cardiovascular side effects of NSAID therapy might be predicted by their effects on potassium channel activators and L-type calcium channel blockers. The regulation of vascular tone, and hence blood pressure, is under the control of a variety of ion channels in vascular smooth muscle cells (VSMCs). More specifically, two types of ion channels are perhaps the most important in determining the contractile state of VSMCs: K+ channels, which are the primary determinants of the resting membrane voltage, and voltage-gated L-type calcium (Ca2+) channels, activation of which allows Ca2+ influx and vasoconstriction57. The effects of the NSAIDs tested in this paper on ion channels have not been studied; therefore, we cannot define how much of the inhibition of contraction might be due to the inhibitory effect of NSAIDs on ion channels.
Our experimental data indicate that NSAIDs decrease NE-induced contraction in aortas from the Control and MS rats. ASA reduces NE-induced contraction by the same proportion in the Control and MS rats at 6 months of age (Figure 3B), even if COX-1 is overexpressed in the MS aortas (Figure 1A). This result could be due to differential activation of COX-1 independent of its expression, an altered presence of the synthases of vasoconstrictor prostanoids or an altered proportion of their receptors in the MS or aged animals. ASA and indomethacin reduced the maximum NE-induced contraction more in the older than younger Control animals (Figure 3B and 3C). This result is consistent with increased COX-1 expression during aging (Figure 1A). Thus, the mechanism of this effect may be COX-1 inhibition, leading to the release of TXA2 and prostaglandin F2α, which are vasoconstricting prostanoids58.
In the arteries of spontaneously hypertensive or diabetic rats, COX-1 expression is up-regulated, and the augmented endothelium-dependent contractions are diminished by COX-1 inhibitors53. Meloxicam caused a decrease in NE constriction, which was greater in the Control old rats than young rats (Figure 3D), suggesting that a COX-2 product is involved and related to age, according to the increase in COX-2 expression during aging (Figure 1B). We have shown up-regulated in the presence of COX-1 and COX-2 in aortas from MS rats at six months of age, which is in accordance with previous results showing that both isoforms can contribute to endothelial dysfunction22,53,59.
In several species, some authors have reported that PLA2 and COX-2 are inflammatory proteins, and their expression is tightly regulated by various mediators60,61,62. PLA2 hydrolyzes membrane phospholipids, resulting in the release of arachidonic acid, which is further converted by COX-2 and prostaglandin synthases to biologically active metabolites22. In accordance with these reports, we found that PLA2 expression is increased in inflammatory conditions, such as MS (at 6 months) and during aging in Control rats.
Experimental studies indicate that endothelium-dependent relaxation to ACh is markedly reduced in aged rat aortas, whereas the response is conserved in other vessels, such as the femoral or mesenteric arteries. In addition, MS is generally considered to induce precocious aging, although the mechanism is not completely known63. A previous report from our group showed that vascular relaxation was decreased in the MS rats31. N-nitro-L-arginine methyl ester (L-NAME), a nonspecific NOS inhibitor, at 300 μmol/L, significantly increased vascular contraction to NE in Control and MS rats at 6 months of age because NOS inhibition induced an imbalance in vasoconstriction and vasodilation that was greater in the MS rats compared to the Control64. Reinforcing this finding, the responses to NE of aortic rings from every age of the Control and MS rats incubated with sodium nitroprusside, an NO donor, did not differ (data not shown). These results demonstrated that MS and aging induced endothelial dysfunction in the aorta, thereby reducing endothelium-induced NO modulation of vasoconstriction.
ACh-induced relaxation involves various overlapping endothelial mechanisms. In some vessels, NO or prostacyclin can produce vascular smooth muscle relaxation or hyperpolarization by activating KATP channels. In SHR and Wistar-Kyoto rat aortas, prostacyclin is the principal metabolite of arachidonic acid released by ACh, with the endothelial cells being the predominant site of its synthesis. Prostacyclin is generally described as an endothelium-derived vasodilator, which, by stimulating its G protein-coupled receptor (prostacyclin receptors), produces smooth muscle relaxation54.
Indomethacin has a beneficial effect on endothelium dependent relaxation in animal models of aging and old patients. However, low-dose aspirin and selective COX-2 inhibitors have been shown to improve or worsen endothelial dysfunction in models of hypercholesterolemia and hypertension21. Hennan et al25 reported that a COX-2–specific inhibitor attenuates arachidonic acid–induced vasodilation in canine coronary arteries, supporting a physiological role for COX-2 in vascular function.
Jung et al26 have reported that a low-dose of aspirin increases the NO produced by blood vessels, but the mechanism responsible for this effect is not fully understood. Aspirin use for cardiovascular diseases increases NOS enzymatic activity in endothelial cell homogenates and platelets, and aspirin at high concentrations acetylates eNOS serine residues. However, our results show that ASA, at 10 μmol/L, is the only NSAID that significantly reduces the response to ACh in NE pre-contracted aortas from young Control rats and old MS rats (Table 3). Future investigations should determine the efficacy of long-term, low-dose treatment with ASA in Control and MS rats.
In conclusion, the present study demonstrates that NSAIDs directly affect vascular responses, and COXs participate in these responses due to differential expression of the isoenzymes. In chronic, low-grade inflammatory conditions, such as MS and aging, COX-2 contributes to a greater extent to vasoconstriction. Thus, understanding the effect of NSAIDs on blood vessels could help improve the treatment of cardiovascular diseases and MS in older people. However, knowing which NSAID is best for a given individual can be difficult. In addition, a person's response to a particular NSAID is hard to predict. The side effects associated with long-term use may aggravate other diseases and even increase morbidity and mortality. There are reports indicating that chronic NSAID use can cause gastrointestinal complaints, and in some cases, the patients have a greater risk of renal impairment and cardiovascular events.
Author contribution
María Esther RUBIO-RUIZ was responsible for planning the experiments, performing the physiological experiments, data analysis and writing; Eulises DIAZ-DIAZ and atalia PAVÓN were responsible for the biochemical measurements; Israel PÉREZ-TORRES was responsible for the Western blot analyses; and Verónica GUARNER-LANS was responsible for planning the experiments, performing the physiological experiments, data analysis and reviewing the manuscript.
Acknowledgments
This work forms part of a PhD thesis for María Esther RUBIO-RUIZ. The authors would like to thank the program of Doctorado en Ciencias Biomédicas, Facultad de Medicina UNAM. We also thank Bertha SOTO, Mario PÉREZ, Edmundo SÁNCHEZ, and Florencio HERNÁNDEZ for technical assistance.
References
- Rubio-Ruiz ME, Guarner-Lans V.Inflammation and the use of anti-inflammatory agents in signs and cardiovascular consequences of metabolic syndrome. In: Lopez-Garcia CM, Perez-Gonzalez PA, editors. Handbook on Metabolic syndrome. Classification, risk factors and health impact. New York: Nova Biomedical; 2012. p169–88.
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin. 2008;29:185–92. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol. 2008;295:H1522–8. doi: 10.1152/ajpheart.00596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Naik JS, Hester RL. Functional vasodilation in the rat spinotrapezius muscle: role of nitric oxide, prostanoids and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol. 2008;35:617–24. doi: 10.1111/j.1440-1681.2007.04864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner-Lans V, Rubio-Ruiz ME. Aging, metabolic syndrome and the heart. Aging Dis. 2012;3:269–79. [PMC free article] [PubMed] [Google Scholar]
- Guarner-Lans V, Rubio-Ruiz ME, Pérez-Torres I, Baños de MacCarthy G. Relation of aging and sex hormones to metabolic syndrome and cardiovascular disease. Exp Gerontol. 2011;46:517–23. doi: 10.1016/j.exger.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, et al. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K-channel and nitric oxide. J Pharmacol Exp Ther. 2011;336:701–8. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- Chennupati R, Lamers WH, Koehler SE, De Mey JG. Endothelium-dependent hyperpolarization-related relaxations diminish with age in murine saphenous arteries of both sexes. Br J Pharmacol. 2013;169:1486–99. doi: 10.1111/bph.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen E, Viskoper JR, Mishal J, Priluk R, London D, Yodefy C. Effects of low-dose aspirin on blood pressure and endothelial function of treated hypertensive hypercholesterolaemic subjects. J Hum Hypertens. 2005;19:667–73. doi: 10.1038/sj.jhh.1001910. [DOI] [PubMed] [Google Scholar]
- Deng S, Deng PY, Jiang JL, Ye F, Yu J, Yang TL, et al. Aspirin protected against enndothelial damage induced by LDL: role of endogenous NO synthase inhibitors in rats. Acta Pharmacol Sin. 2004;25:1633–9. [PubMed] [Google Scholar]
- Heymes C, Habid A, Yand D, Matthieu E, Marotte F, Samuel JL, et al. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–10. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, Cannon PJ. COX-2 Inhibitors and cardiovascular risk. Science. 2012;336:1386. doi: 10.1126/science.1224398. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- McLean A, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2006;56:163–84. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- Sumapa C, Allison JJ, Curtis JR. Risks versus benefits of cyclooxygenase-2-selective nonsteroidal antiinflammatory drugs. Am J Health-Syst Pharm. 2006;63:1837–51. doi: 10.2146/ajhp050519. [DOI] [PubMed] [Google Scholar]
- Wu R, Laplante MA, de Champlain J. Cyclooxygenase-2 inhibitors attenuate angiotensin II-induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension. 2005;45:1139–44. doi: 10.1161/01.HYP.0000164572.92049.29. [DOI] [PubMed] [Google Scholar]
- Bulckaen H, Prévost G, Boulanger E, Robitaille G, Roquet V, Gaxatte C, et al. Low-dose aspirin prevents age-related endothelial dysfunction in a mause model of physiological aging. Am J Physiol Heart Circ Physiol. 2008;294:H1562–70. doi: 10.1152/ajpheart.00241.2007. [DOI] [PubMed] [Google Scholar]
- Raghavan RP, Laight DW, Shaw KM, Cummings MH. Aspirin and diabetes. Br J Diabetes Vasc Dis. 2006;6:74–82. [Google Scholar]
- Von der Weid P, Hollenberg MD, Fiorucci S, Wallace JL. Aspirin-Triggered, cyclooxygenase-2-dependent lipoxin synthesis modulates vascular tone. Circulation. 2004;110:1320–5. doi: 10.1161/01.CIR.0000140985.89766.CB. [DOI] [PubMed] [Google Scholar]
- Wilson SL, Poulter NR. The effect of non-steroideal anti-inflammatory drugs and other commonly used non-narcotic analgesics on blood pressure level in adults. J Hypertens. 2006;24:1457–69. doi: 10.1097/01.hjh.0000239278.82196.a5. [DOI] [PubMed] [Google Scholar]
- Antman EM, De Mets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–70. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- Félétou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WB. Cardiovascular effects of the cyclooxygenase inhibitors. Hypertension. 2007;49:408–18. doi: 10.1161/01.HYP.0000258106.74139.25. [DOI] [PubMed] [Google Scholar]
- Geesaman BJ. Genetics of aging: implications for drug discovery and development. Am J Clin Nutr. 2006;83:466S–469S. doi: 10.1093/ajcn/83.2.466S. [DOI] [PubMed] [Google Scholar]
- Hennan JK, Huang J, Barrett TD, Driscoll EM, Willens DE, Park AM, et al. Effects of selective cyclooxygenase-2 inhibition on vascular responses and thrombosis in canine coronary arteries. Circulation. 2001;104:820–5. doi: 10.1161/hc3301.092790. [DOI] [PubMed] [Google Scholar]
- Jung SB, Kim CS, Naqvi A, Yamamori T, Mattagajasingh I, Hoffman TA, et al. Histone deacetylase 3 antagonizes aspirin-stimulated endothelial nitric oxide production by reversing aspirin-induced lysine acetylation of endothelial nitric oxide synthase. Circ Res. 2010;107:877–87. doi: 10.1161/CIRCRESAHA.110.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baber SR, Champion HC, Bivalacqua TJ, Hyman AL, Kadowitz PJ. Role of cyclooxygenase-2 in the generation of vasoactive prostanoids in the rat pulmonary and systemic vascular beds. Circulation. 2003;108:896–901. doi: 10.1161/01.CIR.0000084536.87322.BB. [DOI] [PubMed] [Google Scholar]
- Baber SR, Hyman AL, Kadowitz PJ. Role of COX-1 and -2 in prostanoid generation and modulation of angiotensin II responses. Am J Physiol Heart Circ Physiol. 2003;285:H2399–410. doi: 10.1152/ajpheart.00294.2003. [DOI] [PubMed] [Google Scholar]
- Qi Z, Cai H, Morrow JD, Breyer MD. Differentiation of cyclooxygenase 1- and 2–derived prostanoids in mouse kidney and aorta. Hypertension. 2006;48:323–8. doi: 10.1161/01.HYP.0000231934.67549.b7. [DOI] [PubMed] [Google Scholar]
- Fosslien E. Cardiovascular complications of Non-Steroidal Anti-Inflammatory Drugs. Ann Clin Lab Sci. 2005;35:347–85. [PubMed] [Google Scholar]
- Rubio ME, Baños G, Diaz E, Guarner-Lans V. Effect of age on insulin-induced endothelin release and vasoreactivity in hypertriglyceridemic and hypertensive rats. Exp Gerontol. 2006;41:282–8. doi: 10.1016/j.exger.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenborough NJ, Farr AL, Randall RJ. Protein measurement with the folin fenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Morley JE. The metabolic syndrome and aging. J Gerontol A Biol Sci Med Sci. 2004;59:139–42. doi: 10.1093/gerona/59.2.m139. [DOI] [PubMed] [Google Scholar]
- Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- Santulli G, Lombardi A, Sorriento D, Anastasio A, del Giudice C, Formisano P, et al. Age-related impairment in insulin release the essential role of β2-adrenergic receptor. Diabetes. 2012;61:692–701. doi: 10.2337/db11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes. 2004;53:441–6. doi: 10.2337/diabetes.53.2.441. [DOI] [PubMed] [Google Scholar]
- Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- Nolte LA, Rincón J, Wahlström EO, Craig BW, Zierath JR, Wallberg-Henriksson H. Hyperglycemia activates glucose transport in rat skeletal muscle via a Ca2+-dependent mechanism. Diabetes. 1995;44:1345–8. doi: 10.2337/diab.44.11.1345. [DOI] [PubMed] [Google Scholar]
- El Haffidi M, Pérez I, Zamora J, Soto V, Carvajal-Sandoval G, Baños G. Glycine intake decreases plasma free fatty acids, adipose cell size and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1387–93. doi: 10.1152/ajpregu.00159.2004. [DOI] [PubMed] [Google Scholar]
- Bollheimer LC, Buettner R, Pongratz G, Brunner-Ploss R, Hechtl C, Banas M, et al. Sarcopenia in the aging high-fat fed rat: a pilot study for modeling sarcopenic obesity in rodents. Biogerontology. 2012;13:609–20. doi: 10.1007/s10522-012-9405-4. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. doi: 10.1155/2013/204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayasi R, Akamine EH, Davel AP. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet induced obesity in mice. J Hypertens. 2010;28:2111–9. doi: 10.1097/HJH.0b013e32833ca68c. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Li JB, Nishida M, Kaimoto K, Asakawa A, Chaolu H, Cheng KC, et al. Effects of aging on the plasma levels of nesfatin-1 and adiponectin. Biomed Rep. 2014;2:152–6. doi: 10.3892/br.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Oh S, Quan S, Ryu OH, Jeong JY, Hong KS, et al. Gender differences in adiponectin levels and body composition in older adults: Hallym aging study. BMC Geriatrics. 2014;14:8. doi: 10.1186/1471-2318-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JCK. Ethnic variability in adiposity and cardiovascular risk: the variable disease selection hypothesis. Int J Epidemiol. 2009;38:63–71. doi: 10.1093/ije/dyn183. [DOI] [PubMed] [Google Scholar]
- Yang R, Barouch LA. Leptin signaling and obesity cardiovascular consequences. Circ Res. 2007;101:545–59. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- Kleint T, Ullrich V, Pfeilschifter J, Nüsing R. On the induction of cyclooxygenase-2, inducible nitric oxide synthase and soluble phospholipase A2 in rat mesangial cells by a Nonsteroideal Anti-inflammatory drug: the role of cyclic AMP. Mol Pharmacol. 1998;53:385–91. doi: 10.1124/mol.53.3.385. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Wang M, Lakatta E, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kB. J Appl Physiol. 2008;105:1333–41. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. Signaling by Gasotransmitters. Sci Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, et al. Cyclooxygenase-2-derived prostaglandin F2a mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–35. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- Gomez E, Schwendemann C, Roger S, Simonet S, Paysant J, Courchay C, et al. Aging and prostacyclin responses in aorta and platelets from WKY and SHR rats. Am J Physiol Heart Circ Physiol. 2008;295:H2198–211. doi: 10.1152/ajpheart.00507.2008. [DOI] [PubMed] [Google Scholar]
- Morgan T, Anderson A. The effect of Nonsteroidal Anti-Inflammatory Drugs on blood pressure in patients treated with different antihypertensive drugs. J Clin Hypertens. 2003;5:53–7. doi: 10.1111/j.1524-6175.2003.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonia J. Interaction of antihypertensive drugs with anti-inflammatory drugs. Cardiology. 1997;88:47–51. doi: 10.1159/000177507. [DOI] [PubMed] [Google Scholar]
- Brueggemann LI, Mani BK, Mackie AR, Cribbs LL, Byron KL. Novel actions of nonsteroidal anti-inflammatory drugs on vascular ion channels: accounting for cardiovascular side effects and identifying new therapeutic applications. Mol Cell Pharmacol. 2010;2:15–9. [PMC free article] [PubMed] [Google Scholar]
- Taubert D, Berkels R, Grosser N, Schröder H, Gründemann D, Schömig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159–65. doi: 10.1038/sj.bjp.0705907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MSK, Man RYK, Vanhoutte PM. Calcium-independent phospholipase A2 plays a key role in the endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2010;298:H1260–6. doi: 10.1152/ajpheart.01068.2009. [DOI] [PubMed] [Google Scholar]
- Beasley D. COX-2 and cytosolic PLA2 mediate IL-1b-induced cAMP production in human vascular smooth muscle cells. Am J Physiol. 1999;276:H1369–78. doi: 10.1152/ajpheart.1999.276.4.H1369. [DOI] [PubMed] [Google Scholar]
- Luo SF, Lin WN, Yang CM, Lee CW, Liao CH, Leu YL, et al. Induction of cytosolic phospholipase A2 by lipopolysaccharide in canine tracheal smooth muscle cells: involvement of MAPKs and NF-kB pathways. Cell Signal. 2006;18:1201–11. doi: 10.1016/j.cellsig.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Lin CC, Lin WN, Wang WJ, Sun CC, Tung WH, Wang HH, et al. Functional coupling expression of COX-2 and cPLA2 induced by ATP in rat vascular smooth muscle cells: role of ERK1/2, p38 MAPK, and NF-kB. Cardiovasc Res. 2009;82:522–31. doi: 10.1093/cvr/cvp069. [DOI] [PubMed] [Google Scholar]
- Barton M. Ageing as a determinant of renal and vascular disease: role of endothelial factors. Nephrol Dial Transplant. 2005;20:485–90. doi: 10.1093/ndt/gfh689. [DOI] [PubMed] [Google Scholar]
- Rubio-Ruiz ME, Díaz-Díaz E, Cárdenas-León M, Argüelles-Medina R, Sánchez-Canales P, Larrea-Gallo F, et al. Glycation does not modify bovine serum albumin (BSA)-induced reduction of rat aortic relaxation: the response to glycated and nonglycated BSA is lost in metabolic syndrome. Glycobiology. 2008;18:517–25. doi: 10.1093/glycob/cwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]