Figure 1.
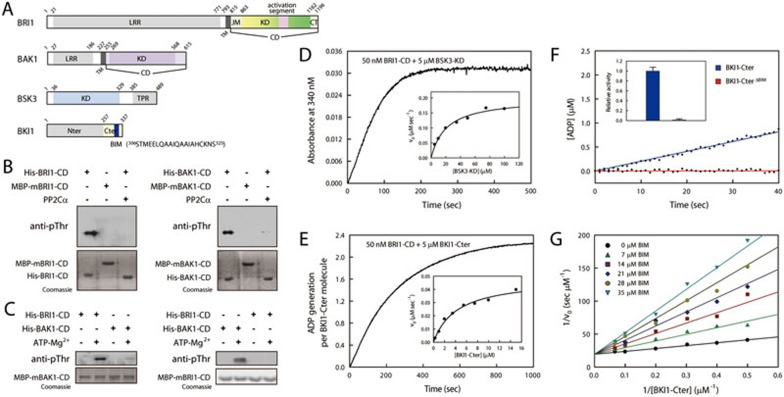
BIM is a major determinant for BRI1-catalyzed BKI1 phosphorylation. (A) Schematic diagram of BRI1, BAK1, BSK3 and BKI1. LRR, leucine-rich repeat domain; TM, transmembrane domain; CD, cytosolic domain; KD, kinase domain; TPR, tetratricopeptide repeat domain; Nter, N-terminal domain; Cter, C-terminal domain; BIM, BRI1-interacting motif. The sequence of the BIM peptide (blue) is provided. (B) Phosphorylation states of recombinant wild-type, kinase-dead mutant and PP2Cα-dephosphorylated BRI1-CD and BAK1-CD analyzed using a general anti-pThr antibody. (C) Transphosphorylation analyses of BRI1-CD and BAK1-CD by western blotting. (D and E) Representative time course of BRI1-CD-catalyzed phosphorylation on BSK3-KD (D) and BKI1-Cter (E). The insets shows the initial rates vs the BSK3-KD and BKI1-Cter concentrations, respectively. (F) Time courses of BRI1-CD-catalyzed phosphorylation of BKI1-Cter and BKI1-CterΔBIM (5 μM). The relative activities of BRI1-CD towards two substrates (mean ± SEM, n = 3) are shown in the inset. (G) Competitive inhibition of BRI1-catalyzed BKI1-Cter phosphorylation by BKI1-BIM. The kinetic parameters were obtained by fitting the experimental data to the competitive inhibition equation. See also Supplementary information, Data S1 and Figure S1.