Abstract
Ficus exasperata have been reported to have wide applications in the treatment of many human diseases. However, its traditional use in the treatment of wounds has not been validated by any scientific study. Also, its safety in the management of chronic disease conditions requires attention. We evaluated the wound-healing activity of the aqueous extract and fractions of F. exasperata, as well as its safety after subchronic oral administration. Similar percentage of wound contraction was observed with 5% w/w extract ointment application and administration of cicatrin powder (standard) on the 4th day, while better contraction than the standard was recorded with higher concentrations of the extract ointment. Of all the fractions tested, significant (P < 0.05) contraction was only noticed in chloroform fraction, though lower than that of the aqueous extract. The extract also showed concentration-dependent inhibition of all the tested microbial isolates. Extract administered up to 5000 mg/kg (single dose administration) did not cause any mortality after 24 h. Mortality was, however, recorded at 4000 mg/kg within the first 20 days of subchronic administration of the extract. Significant (P < 0.05) increases in alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), and in particular, alkaline phosphatase (ALP) were observed at different doses and time periods. Pathological and histological changes were noticed in the liver and kidney on the 91st day of the study with 4000 mg/kg of the extract. Except for the significant (P < 0.05) reduction in WBC on the 91st day, no other significant (P < 0.05) changes were observed in other hematological parameters. The aqueous extract demonstrated better wound-healing activity than its fractions; however, the extract may not be safe at higher doses for subchronic oral administration, as may be the case in the management of chronic disease conditions.
Keywords: Chronic diseases, Ficus exasperata, Hematological parameters, Liver enzymes, Toxicity, Wound healing
INTRODUCTION
Population rise, inadequate supply of drugs, high cost of treatment, side effects, and drug resistance have led to increased use of plant materials as a source of medicine for various human ailments.[1] The valuable medicinal properties inherent in plants have long been acknowledged, as biologically active molecules and lead structures for the development of modified derivatives with enhanced activity have been obtained through various natural product researches.[2] It is not therefore surprising that over three-quarters of the world population rely mainly on plants and plant extracts for health care.[3]
Ficus exasperata, popularly referred to as “sandpaper leaf tree,” finds wide application in ethnomedicine.[4] In African traditional medicine, all the plant parts are considered medicinally important, but the leaves are much valued in the treatment of diseases.[5] The leaf extract has been shown to cause a significant decrease in hyperglycemia, polyurea, and hyperlipidemia and enhanced serum insulin levels in streptozotocin-induced diabetic rats.[6] Reduced blood pressure and restoration of microanatomy of the blood vessels to almost normal levels have also been achieved using the leave extract of F. exasperata in spontaneously hypertensive and obese Zucker rats.[6,7] Hydro-ethanol extract of the leaves of this plant has also been shown to exhibit radical scavenging activity comparable to n-propyl gallate and also inhibits lipid peroxidation in rat brain.[8] Anti-inflammatory,[9] antiarthritic,[8] antinociceptive,[9] anticonvulsant,[10] anxiolytic,[11] antiulcer,[12] antipyretic,[13] and antimicrobial[14] activities of the leaf extract have all been validated in numerous scientific studies.
In south-eastern Nigeria, the leaves are mainly used for the treatment of topical wounds and for the management of diabetes and hypertension. Since no studies have been done to validate its wound-healing activity and its safety in managing chronic ailments, we evaluated the wound-healing activity of the aqueous extract and fractions, as well as its safety after subchronic administration in albino rats.
MATERIALS AND METHODS
Plant materials
Leaves of F. exasperata were collected from Nibo town in Awka South Local Government Area, Nigeria and were authenticated by Dr. J. E. Amadi of the Department of Botany, Nnamdi Azikiwe University, Awka, Anambra state, Nigeria where a voucher specimen (PCG/423A/019) was kept.
Animals
Adult albino rats (150-180 g) were obtained from the animal house, Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University. They were maintained in standard animal house conditions and were fed with growers feed (by Top feeds Nigeria Limited) and water ad libitum. All animal experiments were conducted in compliance with the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Pub. No. 85-23 Revised 1985) and approved by the Nnamdi Azikiwe University, Awka Ethical Committee for the use of laboratory animals.
Methods
Extraction and fractionation
The shade-dried leaves were pulverized, cold macerated for 24 h, and filtered, and the filtrate was concentrated using freeze-drier. Part of the freeze-dried extract (100 g) was adsorbed on silica gel and eluted in succession with n-hexane, chloroform, ethyl acetate, and methanol. The filtrate so obtained was evaporated to a constant weight using rotary evaporator at 40°C.
Qualitative phytochemical analysis
Screening for the presence of secondary metabolites was performed following standard phytochemical tests as described by Harborne.[15]
Antimicrobial evaluation
Agar well diffusion technique, as described by Adeniyi et al.,[16] was used. The bacterial isolates of Proteus mirabilis, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia, and Proteus vulgaris were used. The extract concentrations used were 10, 50, 100, 150, 200, and 250 mg/ml at an equal volume of 1000 μl. Each concentration was tested in triplicate and the zones of inhibition were measured in millimeters.
Wound healing evaluation
Formulation of herbal ointment
The medicated ointment was prepared by incorporating different quantities of the extract or fractions into the ointment base (composed of cetosteryl alcohol, hard paraffin, wool fat, and white soft paraffin) to get 5, 15, 20, and 25% w/w of the extract in ointment. Also 10, and 20% w/w of each fraction in ointment was prepared. The fusion method was adopted in the formulation of the herbal ointment. The required quantity of the ointment base was weighed and melted at a temperature of about 70°C in a water bath.
The designated quantity of the extract (or fraction) was added to the melted base at 40°C in a water bath, stirred gently and continuously until a homogenous dispersion was obtained. The above exercise was repeated using different weights of the crude extract (or fractions) in order to obtain the above-mentioned concentrations.
Wound creation
Thirty adult albino rats divided into six groups of five rats each were used for the study. Excision wounds were created after shaving the left dorsal thoracic region 1 cm away from the vertebral column and 5 cm away from the ear. The animals were anesthetized prior to and during creation of wounds with chloroform using open mask method. Excision wounds of about 3 cm in diameter were created. All wounds were of full thickness, extending down to adipose tissue.[17] The wound areas were measured every 4 days by retracing the wound on a millimeter scale graph paper,[18] cleaned with methylated spirit, and the appropriate ointment concentration applied once a day until complete healing was observed. Wound contraction was calculated as percentage reduction in the wound area with respect to the initial wound area, while epithelialization time was noted as the number of days required after wound infliction for the scab to fall off leaving no raw wounds behind:
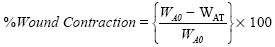
where
WA0= wound area on day 0
WAT= wound area on day T (after induction).
Acute toxicity test
Acute oral toxicity test was conducted as per the Organisation for Economic Co-operation and Development (OECD) guidelines 423.[19] A total of 18 albino mice were divided into three groups of six animals each. The control group received 10 ml/kg of normal saline, while the second and third groups received a single dose of 2000 and 5000 mg/kg of the extract, respectively. The animals were observed for obvious signs of toxicity and mortality at hourly intervals for the next 24 h and thereafter for a total of 14 days.
Subchronic toxicity test
Sixty albino rats were divided into four groups as A, B, C, and D, with each group consisting of 15 rats. After pre-treatment and determination of basal biochemical and hematological parameters, groups B, C, and D received the extract orally at doses of 1000, 2000, and 4000 mg/kg, respectively, daily for 90 days. Group A served as negative control and received 10 ml/kg normal saline. Physical observation of the animals was done on a daily basis.
Five rats from each group were anesthetized using chloroform on the 31st, 61st, and 91st days of the study. Blood samples were collected through retro-orbital plexus and used for the estimation of hemoglobin (Hb), packed cell volume (PCV), red blood cell (RBC) and white blood cell (WBC) count.[20] Serum samples were used for analysis of biochemical markers, i.e. alanine aminotransaminase (ALT),[21] aspartate aminotransaminase (AST),[21] and alkaline phosphatase (ALP).[22] Histological studies were done on liver and kidney isolates from animals of different dose groups on the 91st day.
Statistical analysis
The data were expressed as mean ± SEM. The results were analyzed by SPSS version 19 using one-way analysis of variance. Graphical representation was done using Microsoft Excel 2010. The differences between mean values were considered significant at P < 0.05.
RESULTS
Qualitative phytochemistry
Qualitative phytochemical study of the extract [Table 1] revealed the presence of many phytocompounds. Phlobatannins and terpenoids were found at a high concentration in the chloroform fraction, while alkaloids and saponins were more in the methanol fraction. Ethyl acetate fraction contained saponins, terpenoids, and flavonoids. The n-hexene fraction was more of fats and oil. Due to the low percentage yield of n-hexane fraction, no further study was done with this fraction.
Table 1.
Phytochemical constituents of aqueous extract and fractions of Ficus exasperata

Antimicrobial activities of the extract
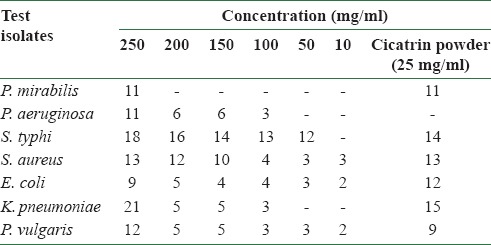
Table 2 shows the results of the antimicrobial activities of different concentrations of the aqueous crude extract on some human pathogens. There was a concentration-dependent inhibition of all the tested micro-organisms. The minimum inhibitory concentration of the leaf extract ranged from 10 to 250 mg/ml.
Table 2.
Inhibition zone diameters (mm) of F. exasperate against test microorganisms
Wound-healing activity
Progressive wound contraction was observed in all the extract-treated groups [Figure 1]. At the lowest concentration of 5% w/w extract ointment, significant (P < 0.05) contraction similar to that of cicatrin powder (standard) was observed on the 4th day. Better contraction than the standard was recorded at higher concentrations of the extract ointment. Epithelialization was observed on the 11th day in the 20% and 25% w/w extract ointment treated groups, while it occurred on the 14th day in the cicatrin powder treated group just like the 15% w/w extract ointment treated group [Table 3]. The group treated with blank ointment took a longer time (24 days) to achieve epithelialization. Less contraction activity was recorded for the fractions [Figure 2]. Only the chloroform fraction (20% w/w) showed significant contraction from the 12th day. Epithelialization time (14 days) for this fraction was, however, the same as that of cicatrin powder.
Figure 1.
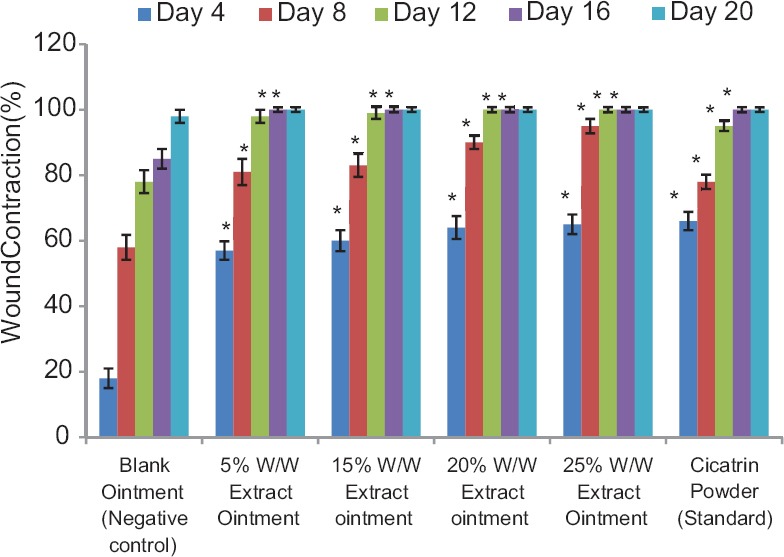
Wound-healing activity of the aqueous leaf extract of F. exasperata.*p < 0.05 compared with control
Table 3.
Epithelialization time of the different formulated extract and fractions ointments
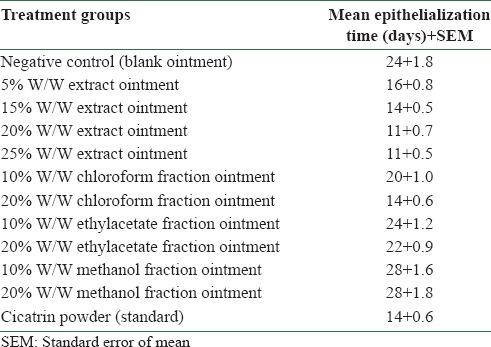
Figure 2.

Wound-healing activity of different fractions of aqueous leaf extract of F. exasperata. *P < 0.05 compared with control
Acute toxicity test
No obvious physical and behavioral side effect was observed. Up to 5000 mg/kg, the extract did not cause any mortality after 24 h and subsequently for 14 days. The LD50 was, therefore, estimated to be greater than 5000 mg/kg.
Subchronic toxicity tests
By day 17 of daily oral administration of the extract, obvious signs of weakness were observed in some of the rats that received 4000 mg/kg of the extract and, subsequently, eight rats died in this group within the first 20 days. Thereafter, no death was recorded in all the groups. Significant (P ˂ 0.05) increase in ALT was observed only for the highest dosed group on the 61st and 91st days [Figure 3a]. Groups that received 2000 and 4000 mg/kg of the extract exhibited significant (P < 0.05) increase in AST on the 61st day, while significant (P < 0.05) increase was observed with all doses on the 91st day [Figure 3b]. Also, significant (P < 0.05) increase in ALP was observed in all the extract-treated groups on the 61st and 91st days. It was also observed that increase in ALP in the group treated with 4000 mg/kg became significant (P < 0.05) even on the 31st day [Figure 3c].
Figure 3.
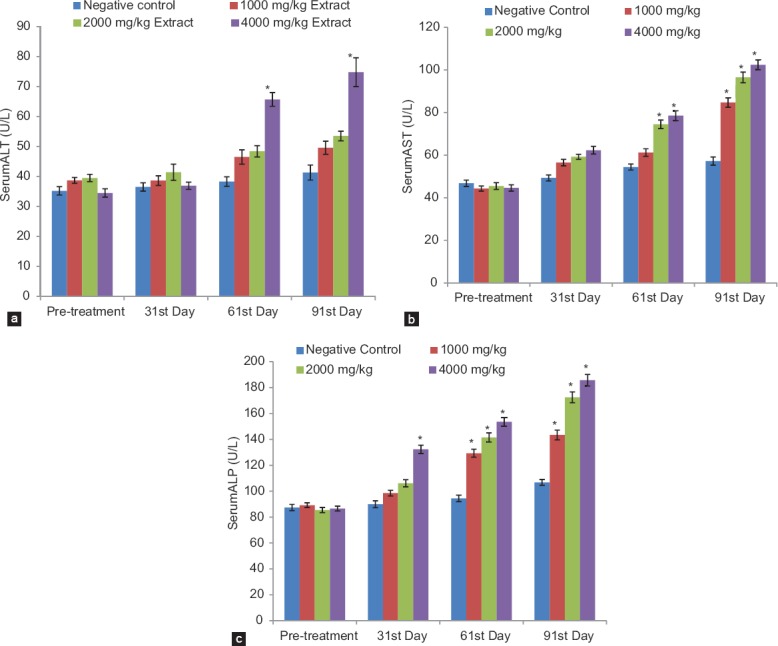
Effects of extract on liver enzymes: (a) Alanine aminotransaminase, (b) Aspartate aminotransaminase and (c) Alkaline phosphatase. *p < 0.05 compared with control
Histological changes in the liver of the highest dosed group correlated with the biochemical changes observed. Histology of the liver in this group showed proliferation of the tissue stroma, edema, dilatation, hemorrhage, and increased size of the hepatocytes [Figure 4]. Histology of the kidney of negative control revealed intact glomeruli. As the dose of the extract was increased (1000, 2000, and 4000 mg/kg), there was a progressive decrease in the number of glomeruli [Figure 5]. Changes in the kidney histology became obvious in the group that received 4000 mg/kg b.wt. of the extract. Most glomeruli were either shrunken or lost, in addition to tubular hemorrhage occurring in this group. Hematological analysis revealed no significant (P ˃ 0.05) changes in PCV [Figure 6a], Hb [Figure 6b], and RBC [Figure 6c] values in all the groups. Significant (P ˂ 0.05) decrease in the WBC was observed in all the extract-treated groups on the 91st day [Figure 6d].
Figure 4.
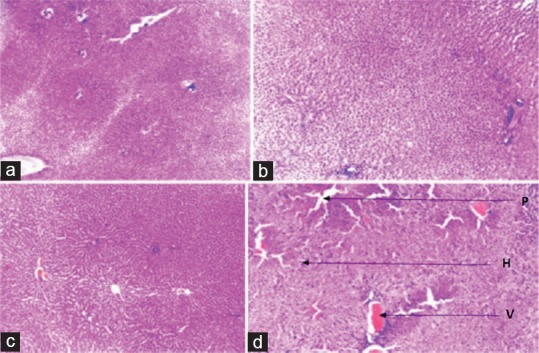
Liver histology. (a) Negative control, with normal liver histology, (b) no significant changes observed with 100 mg/kg, (c) proliferation of the tissue observed with 2000 mg/kg, (d) proliferation of the tissue stroma, oedema (p), dilatation and haemorrhage within the central vein (v), increase in size of the hepatocytes (h) observed with 4000 mg/kg
Figure 5.
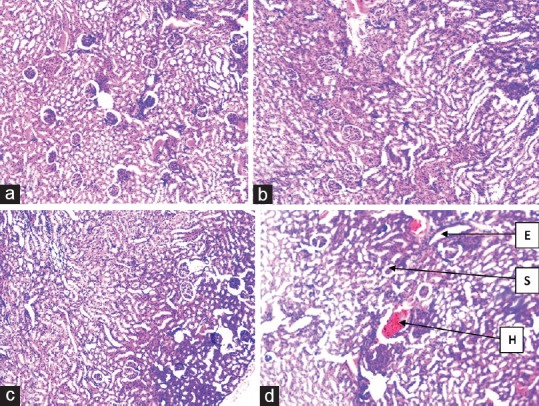
Kidney histology. (a) Negative control with normal features, (b) 1000 mg/kg of extract with no significant change, (c) 2000/kg of extract with no significant change, (d), 4000 mg/kg of extract showing significant damages: oedema (H), shrunken /loss of glomeruli(S), haemorrhage (H), tissue stromal distortion(S)
Figure 6.

Effects of extract on haematological parameters: (a) Packed cell volume (PCV), (b) Hemoglobin (HB), (c) Red blood cell (RBC) and (d) White blood cell (WBC). *p < 0.05 compared with control
DISCUSSION
Wounds provide a conducive environment for the growth of micro-organisms. Usually, microbial contaminants of wounds involve a variety of organisms such as P. aeruginosa, Sta. aureus, Streptococcus faecalis, E. coli, Clostridium perfringens, Clostridium tetani, coliform bacilli, and enterococcus.[23,24] Evaluation of the extract in clinically isolated microbial contaminants of wounds showed dose-dependent inhibitory activity against some pathogenic micro-organisms including P. aeruginosa and Sta. aureus, two organisms mostly implicated in chronic and non-healing wounds.[25] Microbial infection in wound delays healing and causes more pronounced acute inflammatory reaction which can lead to further tissue injury and damage.[24] Thus, the antimicrobial activity of the extract on these wound isolates may partly contribute to the wound-healing effect by eliminating infection and thus allowing initiation of natural tissue repair processes. It also suggests the leaf extract may play useful role in accelerating the healing of old wounds by eradicating already established infection.
Phytochemical analysis of the extract and fractions revealed the presence of alkaloids, saponins, sterols, terpenoids, glycosides, tannins, and flavonoids. These metabolites are usually responsible for the pharmacological activities of medicinal plants.[26] Saponins and flavonoids have been reported to possess wound-healing activity.[27] Terpenoids are known to promote wound-healing process, mainly due to their astringent and antimicrobial activities which seem to be responsible for wound contraction and increased rate of epithelialization.[28] Flavonoids and their derivatives are known to decrease lipid peroxidation by improving vascularity leading to slowing down of cell necrosis.[29] Phlobatannins have also been demonstrated to have wound-healing activity.[30] Therefore, the wound-healing potential of F. exasperata can be attributed to the contributions of individual phytoconstituents. Synergistic or complementary effects of the phytoconstituents were further demonstrated by higher activity recorded by the extract compared with the fractions. Significant activity recorded by the chloroform fraction indicates that phlobatannins may have played a significant role in the overall wound-healing activity of the extract.
Conventional wound treatment requires, more or less, the combined effects of antibiotics, anti-inflammatory agents, astringents, and antipyretics; F. exasperata has been reported to possess antibacterial,[14] anti-inflammatory,[9] antioxidant,[8] and antipyretic effects.[13] The extract can, therefore, be effectively used in the treatment of wounds.
Single administration of the extract up to 5000 mg/kg did not produce any sign of toxicity or mortality, an indication that may have led to the assumption that the extract is safe. However, this was not the case observed on subchronic administration, as obvious signs of toxicity and mortality were recorded within the first 20 days in the highest dosed group. These signs alerted us of the inherent toxicity that may be associated with this extract.
ALT, AST, and ALP are the enzymes associated with liver function and are an indirect measure of liver homeostasis.[31] Elevation of these marker enzymes is an indication of hepatotoxicity associated with the extract. It was observed that elevation of ALP preceded that of other marker enzymes. ALP elevation is usually associated with cholestasis due to biliary obstruction or hepatic infiltration,[32] which may be a key to hepatotoxicity associated with this extract. Biochemical alterations observed were complemented by histological changes in the liver and kidney. This is in line with the observed increases in liver and kidney weights as well as serum liver enzymes reported following acute administration of aqueous extract of F. exasperata.[33] Also, Bwititi et al.[34] and Ijeh and Ukweni[35] have indicated toxic injury to the kidney following acute exposure to F. exasperata. The toxicity of F. exasperata leaf has been attributed to its high content of cyanogenic glycosides[36] and may have contributed to the observed toxicity recorded in this study.
Hematological system is highly susceptible to drug-induced toxicity due to its vital position and functions.[37] The reduction in WBC on the 91st day in all the extract-treated groups may be due to the direct destructive effect of the extract on these cells or their impaired production in the hematopoietic tissues (bone marrow). Moreover, the reduction of WBC following extract treatment might be because of the mobilization of the leukocytes to the tissues surrounding the blood.
CONCLUSION
The present study justified the use of the leaf extract of F. exasperata in the treatment of cutaneous wounds more than any of its fractions. However, F. exasperata may not be safe at higher doses, especially for the management of chronic disease conditions like hypertension and diabetes, as could be observed in the subchronic toxicity and histopathology findings.
REFERENCES
- 1.Yogayata SP, Vijay DW. Herbal medicines and nutritional supplements used in the treatment of Glaucoma. Res J Pharm Biol Chem Sci. 2012;3:331–9. [Google Scholar]
- 2.Sundaram R, Jacob Inbaneson S, Suganthi P. In vitro antiplasmodial activity of ethanolic extracts of South Indian medicinal plants against Plasmodium falciparum. Asian Pac J Trop Dis. 2012;2:180–3. [Google Scholar]
- 3.Yogisha S, Raveesha KA. In vitro antibacterial effect of selected medicinal plant extracts. J Nat Prod. 2009;2:64–9. [Google Scholar]
- 4.Ahmed F, Mueen Ahmed KK, Abedin MZ, Karim AA. Traditional uses and pharmacological potential of Ficus exasperata vahl. Syst Rev Pharm. 2012;3:15–23. [Google Scholar]
- 5.Akah PA, Orisakwe OE, Gamaniel KS, Shittu A. Evaluation of Nigerian traditional medicines: II. Effects of some Nigeria folk remedies on peptic ulcer. J Ethnopharmacol. 1998a;62:123–7. doi: 10.1016/s0378-8741(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 6.Adewole SO, Adenowo T, Naicker T, Ojewole JA. Hypoglycaemic and hypotensive effects of Ficus exasperata Vahl. (Moraceae) leaf aqueous extract in rats. Afr J Tradit Complement Altern Med. 2011;8:275–83. doi: 10.4314/ajtcam.v8i3.65290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayinde BA, Omogbai EK, Amaechina FC. Pharmacognosy and hypotensive evaluation of Ficus exasperata Vahl (moraceae) leaf. Acta Pol Pharm. 2007;64:543–6. [PubMed] [Google Scholar]
- 8.Abotsi WM, Woode E, Ainooson GK, Amo-Barimah AK, Boakye-Gyasi E. Antiarthritic and antioxidant effects of the leaf extract of Ficus exasperata P. Beauv. (Moraceae) Pharmacognosy Res. 2010;2:89–97. doi: 10.4103/0974-8490.62958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woode E, Poku RA, Ainooson GK, Boakye-Gyasi E, Abotsi WK, Mensah TL, et al. An evaluation of the anti-inflammatory, antipyretic and antinociceptive effects of Ficus exasperata (Vahl) leaf extract. J Pharmacol Toxicol. 2009;4:138–51. [Google Scholar]
- 10.Woode E, Poku RA, Abotsi WK. Anticonvulsant effects of leaf extract of Ficus axasperata Vahl (Moraceae) in mice. Int J Pharmacol. 2011;7:405–9. [Google Scholar]
- 11.Woode E, Poku RA, Abotsi WK. Anxiolytic-like effects of leaf extract of Ficus axasperata Vahl (Moraceae) in mice. West Afr J Pharm. 2011;22:75–81. [Google Scholar]
- 12.Akah PA, Orisakwe OE, Gamaniel KS, Shittu A. Evaluation of Nigerian Traditional medicines on peptic ulcer. J Ethnopharmacol. 1998;62:123–7. doi: 10.1016/s0378-8741(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 13.Bafor EE, Uwumarongie HO, Idiake JO. Antipyretic effects of the aqueous, ethylacetate and hexane leaf extracts of Ficus exasperata Moraceae) in mice. J Thermal Biol. 2010;35:275–9. [Google Scholar]
- 14.Adebayo EA, Ishola OR, Taiwo OS, Majolagbe ON, Adekeye BT. Evaluation of the methanol extract of Ficus exasperata stem bark, leaf and root for Phytochemical analysis and antimicrobial activities. Afr J Plant Sci. 2009;3:283–7. [Google Scholar]
- 15.Harbourne JB. 3rd ed. London: Chapman and Hall; 1998. Phytochemical methods: A guide to modern techniques of plant analysis; p. 302. ISBN: 0-412-572770-2. [Google Scholar]
- 16.Adeniyi BA, Odelola HA, Oso BA. Antimicrobial potentials of Diospyros mespiliformis (Ebenaceae) Afr J Med Sci. 1996;25:221–4. [PubMed] [Google Scholar]
- 17.Morton JJ, Malone MH. Evaluation of vulnerary activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196:117–26. [PubMed] [Google Scholar]
- 18.Reddy JS, Rao PR, Reddy MS. Wound healing effects of Haliotropium indicum, Plumbago zeylenicum and Acalypha indica in rats. J Ethinopharmacol. 2002;79:249–51. doi: 10.1016/s0378-8741(01)00388-9. [DOI] [PubMed] [Google Scholar]
- 19.OECD Guidelines for the Testing of Chemicals (No 423) “Acute Oral Toxicity-Acute Toxic Class Method” (Adopted on 17 December, 2011) [Google Scholar]
- 20.Kelly F. London: Baller Tindall; 1977. Veterinary Clinical Diagnosis; pp. 271–82. [Google Scholar]
- 21.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 22.King EJ, Armstrong AR. A convenient method for determining serum and bile phosphatase activity. Can Med Assoc J. 1934;31:376–81. [PMC free article] [PubMed] [Google Scholar]
- 23.Emele FE, Izomoh MI, Alufohia E. Microorganisms associated with wound infections in Ekpoma, Nigeria. West Afr J Med. 1999;18:97–100. [PubMed] [Google Scholar]
- 24.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14:244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjamsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 26.Nair R, Kalariya T, Chanda S. Antibacteria activity of some selected Indian medicinal flora. Turk J Biol. 2005;29:41–7. [Google Scholar]
- 27.Jian PS, Bari SB. Evaluation of wound healing effect of petroleum ether and methanolic extract of Abemoschus manihot, Medikik malvaceae, Wrightia tinctoria R. Br. Apocyanacae in rats. Braz J Pharmacogn. 2010;20:156–271. [Google Scholar]
- 28.Scortichini M, Pia Rossi M. Preliminary in vitro evaluation of the antimicrobial activity of terpenes and torpenoids towards Erwinia amylovora (Burrill) J Appl Microbiol. 1991;71:109–12. [Google Scholar]
- 29.Manjunatha BK, Vidya SM, Rashmi KV, Mankani KL, Shilpa HJ, Singh S, Singh SD. Evaluation ofwound healing potency of Vernonia arborea HK. Indian J Pharmacol. 2005;37:223–6. [Google Scholar]
- 30.Kagbo H, Ejebe D. Phytochemistry and preliminary toxicity studies of the methanol extract of the stem bark of Garcinia kola (Heckel) Internet J Toxicol. 2009;7:2. [Google Scholar]
- 31.Ilodigwe EE, Okoye GO, Mbagwu IS, Agbata CA, Ajaghaku DL. Safety evaluation of ethanol leaf extract of Picralima nitida stapf (Apocynaceae) Int J Pharmacol Ther. 2012;2:6–17. [Google Scholar]
- 32.Wiwanitkit V. High serum alkaline phosphatase levels, a study in 181 Thai adult hospitalized patients. MBC Fam Pract. 2001;2:2. doi: 10.1186/1471-2296-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irene II, Chukwunonso CA. Body and organ weight changes following administration of aqueous extracts of Ficus exasperata. Vahl on white albino rats. J Anim Vet Adv. 2006;5:277–9. [Google Scholar]
- 34.Bwititi P, Musabayane CT, Nhachi CF. Effects of Opuntia megacantha on blood glucose and kidney function in streptozotocin diabetic rats. J Ethnopharmacol. 2000;69:247–52. doi: 10.1016/s0378-8741(99)00123-3. [DOI] [PubMed] [Google Scholar]
- 35.Irene II, Iheanacho UA. Acute effect of administration of ethanol extracts of Ficus exasperata vahl on kidney function in albino rats. J Med Plant Res. 2007;1:27–9. [Google Scholar]
- 36.Abbiw T. Study of tropical shrubs and plant. J Biogeorge. 1990;23:591–602. [Google Scholar]
- 37.Yao L, Zay YO, Shu YC, Peng H, Kim GE, Jia LZ, et al. An in vitro method for the prediction of renal proximal tubular toxicity in humans. Toxicol Res. 2013;2:352–65. [Google Scholar]


