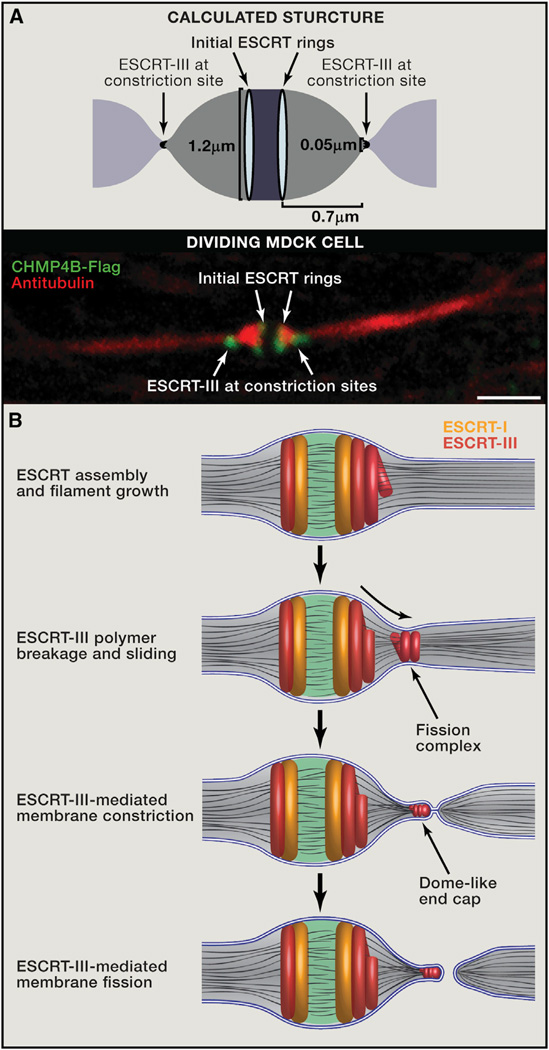
Figure 3. Mechanistic Model for ESCRT-Mediated Abscission.
(A) The calculated shape of the intercellular bridge from computational modeling (top) and the morphology of the bridge as observed by tubulin and ESCRT-III staining using spinning disk confocal microscopy in dividing MDCK cells (bottom) are very similar. The model predicts that when the bridge diameter is 1.2 µm, the optimal distance between the initial and late ESCRT-III pools will be 0.7 µm, which is similar to the distance measured experimentally. Scale bar, 2 µm.
(B) Suggested mechanistic model for ESCRT-driven cytokinetic abscission based on high-resolution microscopy data and computational modeling. As detailed in the text, cytokinetic abscission begins with the assembly of early and late ESCRT proteins into a series of partially overlapping cortical rings located at the center of the intercellular bridge. Ring formation is followed by ESCRT-III polymerization and remodeling into 3D helical spirals. Breakage and sliding of the membrane-associated ESCRT-III spiral away from the dark zone, results in acute constriction of the cytokinetic tube. This continues until the ESCRT-III spiral reaches an equilibrium distance where it relaxes to a spontaneous diameter of 50 nm. At this point fission of the 50 nmdiameter constricted membrane tube occurs, mediated by a dome-like end-cap structure, finalizing cell separation. Similar events occur on the other side of the bridge (not illustrated).