Table 1.
Optimization of conditions for oxidative [3+2] cycloaddition
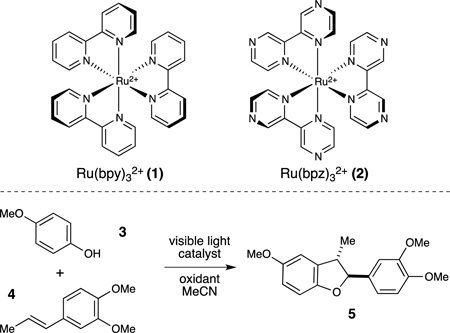 | |||
|---|---|---|---|
| Entry[a] | Catalyst | Oxidant | Yield[b] |
| 1 | Ru(bpy)3(PF6)2 | H2O2 (30% aq) | 0% |
| 2 | Ru(bpy)3(PF6)2 | H2O2•urea | 0% |
| 3 | Ru(bpy)3(PF6)2 | t-BuOOH | 0% |
| 4 | Ru(bpy)3(PF6)2 | m-CPBA | 0% |
| 5 | Ru(bpy)3(PF6)2 | Oxone® | 7% |
| 6 | Ru(bpy)3(PF6)2 | K2S2O8 | 20% |
| 7 | Ru(bpz)3(PF6)2 | K2S2O8 | 75% |
| 8 | Ru(bpz)3(PF6)2 | Na2S2O8 | 23% |
| 9 | Ru(bpz)3(PF6)2 | (NH4)2S2O8 | 78% |
| 10c | Ru(bpz)3(PF6)2 | (NH4)2S2O8 | 0% |
| 11 | none | (NH4)2S2O8 | 0% |
All reactions were irradiated for 24 h using 0.10 mmol phenol, 0.13 mmol styrene, 0.20 mmol oxidant, and 0.005 mmol catalyst, unless noted.
Yields determined by 1H NMR spectroscopy using TMSPh as an internal standard.
Reaction performed in the dark.
