Abstract
T helper 17 (TH17) cells have been identified as a new lineage of helper T cells and have been shown to be important in host defense against extracellular infectious agents, autoimmune disease and chronic inflammatory diseases. Recently, TH17 cells have also been shown to participate in successful pregnancy, as well as in the pathogenesis of diseases of pregnancy, such as recurrent spontaneous abortion (RSA) and pre-eclampsia (PE). Here, we review our current knowledge of TH17 cells in human RSA and PE. We also discuss how the local uterine microenvironment affects the differentiation of TH17 cells and the mechanisms that regulate TH17 cells during pregnancy. Research into TH17 cells will not only advance our understanding of TH17-related pregnancy complications, but will also facilitate the design of novel therapies for reproductive diseases.
Keywords: TH17 cells, human pregnancy, recurrent spontaneous abortion, pre-eclampsia
Introduction
T helper 17 (TH17) cells are a novel lineage of CD4+ T helper cells that have been shown to be important in autoimmunity and the clearance of mucosal infections through the production of interleukin (IL)-17A, IL-17F and IL-22, These cytokines then induce a massive reaction due to the widespread distribution of IL-17 and IL-22 receptors.1,2,3 TH17 cells have been shown to be important in numerous autoimmune and chronic inflammatory diseases, both in humans and experimental animals, and they play a crucial role in host defenses that protect against extracellular fungi, bacteria and Mycobacterium tuberculosis.4,5 When pathogens invade mucosal tissues, IL-17 induces the production of antimicrobial proteins, such as human beta-defensin 2, lipocalin and the calgranulins, as well as CXC chemokines and granulocyte colony-stimulating factor, which results in protection from bacterial infection.6 The most interesting data on the actions of TH17 cells are derived from studies of multiple sclerosis in humans and in its animal model, experimental autoimmune encephalomyelitis (EAE).3,7 TH17 cells have been shown to play a pivotal role in the pathogenesis of EAE, which is an inflammatory disease that involves autoimmune T cell targeting of myelin components. Mice deficient in the thymus-specific isoform of retinoic acid receptor-related orphan receptor gamma T (RORgt) lack tissue-infiltrating TH17 cells and exhibit attenuation of experimentally induced autoimmune disease.8 TH17 cells have a rapid response at the site of inflammation, bridging the gap between innate and adaptive immunity. Some populations of CD8+ T cells, natural killer (NK) cells, gamma delta T cells, neutrophils, eosinophils, monocytes and innate lymphoid cell have also been identified as producers of IL-17;9,10,11 however, the main producer of IL-17A in human is CD4+ TH17 cells.
Recent data show that TH17 cells participate in pregnancy-related pathologies, including recurrent spontaneous abortion (RSA)12,13,14 and pre-eclampsia (PE),15,16 and imbalances between TH1/T regulatory (Treg)/TH17 subsets in both circulation and uterus have been reported.6,17 In this review, we summarize the current knowledge of TH17 cells and the role of this type of cell in pregnancy and specifically in human RSA and PE. In addition, we discuss recent studies that have shown how the uterine local microenvironment affects the differentiation of TH17 cells and the mechanisms that regulate TH17 cells during pregnancy. We argue that research into TH17 cells will facilitate the design of novel therapies to manage patients with pregnancy-related disease.
TH17 cells in human pregnancy diseases
Tom Wegmann was the first investigator to propose that the TH1/TH2 paradigm of pregnancy is critical for fetal survival.18 This idea was reinforced by the observations that a TH2 bias fails to develop or is reversed, resulting in the predominance of TH1 responses in pregnancy complications, including recurrent miscarriage and PE occur.19,20 However, recent findings have shown that this concept requires re-evaluation. In allogeneic mouse pregnancy models, even in the absence of IL-5, IL-9 and IL-13, normal pregnancies are experienced.21 In contrast, neutralization of the prototypical TH1 cytokine interferon gamma (IFNG) results in more pronounced tissue injury during pregnancy. Anne Croy's group has shown that IFNG has an important role in triggering the process of gestational spiral arterial modification and initiating decidual integrity and uterine NK cell maturation during normal murine pregnancy.22,23 Therefore, the bias towards TH2 cytokines may be a physiological deviation that is not essential to the success of a pregnancy.24
Recently, many groups have reported that TH17 cells are present under many circumstances, including normal pregnancy, RSA and PE. In normal pregnancy, most of the IL-17-producing cells are CD4+ T cells in the peripheral blood and decidua.25,26 The proportion of IL-17+ lymphocytes in the decidua is significantly higher than that in the peripheral blood in 1st trimester pregnant women.25,27 However, it is still uncertain whether TH17 cell levels are stable during pregnancy or decrease in late pregnancy based on data from different groups. In addition, the major source of IL-17 is still unclear. Some groups have reported that IL-17+ cells are localized to the glands and the basal proliferative stromal cells in mice.28 Other investigators have reported that IL-17-positive staining is restricted to lymphocytes.26 In humans, it has been suggested that IL-17 can increase the invasive capacity of JEG-3 cells (a trophoblast-like human choriocarcinoma cell line) and increase progesterone secretion significantly in in vitro coculture models.29,30 Additionally, in the third trimester of normal pregnancy, serum IL-17 levels increase in healthy women, which suggests that increased IL-17 might be involved in labor and/or inflammation.31 These evidences suggest that TH17 cells might be very important and helpful in establishing pregnancy. However, more data from in vivo experiments and human studies are needed to verify whether the appearance of TH17 cells is a cause or resultant outcome from establishing a pregnancy.
It has been reported that TH17 cells have an important role in allograft rejection.32,33 Upregulation of IL-17 and IL-23 has been observed at the site of rejection and in draining lymph nodes.34 Similarly, the semi-allogeneic fetus invades the maternal host as an allograft during pregnancy; therefore, it is of great interest to investigate TH17 cells in cases of pregnancy loss. RSA is defined as two or more consecutive pregnancy losses, a condition that affects 1% of all women. RSA due to unknown causes is called unexplained recurrent spontaneous abortion. In unexplained RSA patients, it has been reported that the proportion of TH17 cells and the amount of the concentrations of IL-23, the TH17-inducing cytokine, are higher in the peripheral blood and deciduas than in normal pregnancies. Similarly, these tissues have elevated levels of RORC, an essential transcription factor in TH17 cells.14 In addition, Treg cells, which promote gestational tolerance, are decreased in unexplained RSA.35,36 Estrogen and placental protein 14 have recently been shown to induce Treg cell differentiation and reduce the secretion of IL-17.37,38 Whether estrogen and placental protein 14 are involved in the reciprocal differentiation between TH17 cells and Treg cells in RSA needs further exploration. Importantly, pro-inflammatory cytokines, such as IL-6 and IL-1beta, have also been shown to be increased, and they may also participate in the differentiation of TH17 cells in cases of unexplained RSA.17
PE is a major cause of maternal and neonatal mortality that occurs in 3%–10% of all pregnancies. PE is characterized by the development of maternal high blood pressure (>140/90 mmHg) and proteinuria (>300 mg/24 h) in the second half of pregnancy.39 Although the precise mechanisms leading to the development of PE remain unknown, there is evidence that chronic inflammation, poor angiogenesis and shallow extravillous trophoblast invasion into the uterine spiral arteries as well as inadequate tolerance are associated with the pathogenesis of PE.40,41 Recently, in the pregnant rat model of low-dose lipopolysaccharide (LPS), abnormal inflammation was identified as associated with deficient trophoblast invasion and deficient spiral artery remodeling, and with renal structural alterations and proteinuria characteristic of PE.42 Santner-Nanan et al.27 reported that the size of peripheral blood TH17 cells decreased in normal pregnancy, but in PE cases, this change was not observed. An imbalanced change in T-cell transcription factors was reported in PE in mononuclear cells from both peripheral blood and the decidua. Expression of the master gene for Treg differentiation FOXP3 was decreased, while expression of the TH1 cell transcription factor Tbet and the TH17 cell transcription factor RORC was significantly increased, suggesting that a predominantly TH17 and TH1 type response predominates and there is decreased Treg immunity in PE.43 It has been reported that soluble endoglin, an anti-angiogenic protein, acts together with soluble fms-like tyrosine kinase 1 and placental growth factor, to herald the onset of PE in pregnant rats and humans. Soluble endoglin also acts as an inhibitor of transforming growth factor (TGF)-beta-receptor signaling; therefore, the increased expression of endoglin may result in the expansion of TH17 cell populations and a reduction in the number of Treg cells in PE.44 Therefore, the immune adaptation theories of poor angiogenesis, endothelial dysfunction and chronic inflammation of the pathophysiology of PE might be linked by unbalanced differentiation between Treg and TH17 cells.16 In addition, the predominantly TH1-type immunity reported in PE demonstrates that TH1-dominant immunity induces increased inflammatory cytokines such as IL-6 and IL-1beta, which may further induce the differentiation of TH17 cells.45,46
Systemic and local priming of TH17 cell differentiation in pregnancy
Several differentiation factors and transcription factors that are unique to TH17 cells have been identified, marking TH17 cells as an independent subset of T helper cells. TGF-beta and IL-6 have been reported as the minimal requirements in mice for TH17 cell differentiation from naive CD4+ T cells; in contrast, IL-1beta plus IL-6 or IL-23 are required in human TH17 cells.47,48,49,50,51,52 IL-23/IL-23R plays an important role in stabilizing and endowing TH17 cells with pathogenic effector functions, that are regulated by serum glucocorticoid kinase 1.53 Compared with other TH lineages, including TH1, TH2 and Treg cells, TH17 cells have unique genetic programs to express the transcription factor RORgt, which induces the transcription of the Il17A gene.8 Other transcription factors, such as STAT3,54 RORalpha55 and interferon regulatory factor 4,56 have been reported to be important in TH17 differentiation. Aryl hydrocarbon receptor, an environmental toxin sensor, has also been identified as a regulator of TH17 cytokines, especially IL-22 production.57,58
In pregnancy, the fetus is similar to an allograft from the perspective of the maternal immune system. Trophoblast invasion from the allogeneic fetus and the shedding of fetal antigens may stimulate a maternal systemic inflammatory response and may therefore cause the emergence of TH17 cells. Contrary to Wegmann's hypothesis, it is surprising that many of the characteristics of a systemic inflammatory response have been demonstrated in normal pregnant women,59 including increased leukocytes,60 monocytic61 and phagocytic activity62 and the production of pro-inflammatory cytokines, such as IL-6, IL-12, IL-18 and TNF-alpha.63,64 A classical marker of inflammatory activity, C-reactive protein, is increased beginning as early as the fourth week of gestation.65 These phenomena show that pregnancy is a well-controlled systemic inflammatory state. Furthermore, it has been reported that subcellular microparticles that are shed from the placenta are present during normal pregnancy and are increased significantly in PE.66,67 These subcellular microparticles shed from the placenta are pro-inflammatory and can stimulate peripheral blood mononuclear cells in healthy non-pregnant females to produce TNF-alpha, IL-12, IL-18 and IFNG. Therefore, such microparticles might contribute to the maternal systemic inflammation observed in both normal and pre-eclamptic pregnancies.68 In addition, the microparticles, cellular debris and exosomes shed by the allogeneic fetus can be captured by antigen-presenting cells, contribute to the priming of fetal-reactive T cells during pregnancy.69 However, whether these fetal antigens have a direct relationship with the differentiation of TH17 has not yet been resolved.
We and others have observed low numbers of TH17 cells and low levels of the TH17-related mRNAs RORC and IL-23R in the deciduas during normal human pregnancy.25,70 In RSA, increased levels of inflammatory cytokines, including IL-6 and IL-1beta, have been observed, and these cytokines have a positive correlation with the proportion of TH17 cells.12,70 The inflammatory stimulus from local allogeneic fetal antigens, systematic inflammation and increased pro-inflammatory cytokines may induce the differentiation of TH17 cells during pregnancy.
The regulation towards TH17 cells
Several mechanisms are involved in the regulatory activity of TH17 cells. NK cells are innate immune lymphocytes that play a critical role in regulation of adaptive immune responses, as well as in controlling several types of tumors and microbial infections. Studies of EAE indicate that NK cells inhibit myelin-reactive T cells and control inflammation in the central nervous system in apparent contrast with recent studies in human multiple sclerosis models.71 Several mechanisms have been shown to be involved in TH17 cell regulation in the EAE model. First, it has been suggested that NK cells in the central nervous system directly lyse microglia and suppress myelin-reactive TH17 cells. Because macrophages acting as antigen-presenting cells are crucial in initiating and propagating pathogenic T-cell responses during EAE, microglia appear to become targets of recruited activated NK cells, resulting in a reduced number of myelin-reactive TH17 cells in the central nervous system.72 Second, NK cells may exert a direct cytotoxic effect on auto-antigen-specific encephalitogenic T cells.73 In vitro experiments show that NK cell activity directly results in the lysis of cocultured proteolipid protein peptide-specific encephalitogenic T cells. Third, NK cells may inhibit the T-cell proliferation triggered by antigenic or cytokine stimulation during EAE.74
Distinct from the CD56dimCD16+CD27−CD11b+ NK phenotype in peripheral blood, CD56brightCD16− decidual NK cells comprise approximately 70% of all decidual lymphocytes and act as key regulatory cells at the maternal–fetal interface.75 Both mouse and human studies suggest that these decidual NK cells regulate vascular remodeling and trophoblast invasion, promote tolerogenic dendritic cells and monocytes and suppress TH17-mediated local inflammation, thereby maintaining immune balance and the pregnancy itself.76 Our group recently demonstrated that decidual NK cells function as key regulatory cells at the maternal–fetal interface by suppressing TH17-mediated local inflammation via IFNG-dependent pathways. The proportion of TH17 cells is significantly increased in RSA deciduas in both mice and humans. In women with RSA, the NK cell-mediated regulatory response is lost, resulting in a prominent TH17 response, extensive local inflammation and the eventual loss of maternal–fetal tolerance.70 Although the exact cellular and molecular mechanisms that shape NK cell phenotypes and functions at the maternal-fetal interface still need to be determined, it appears that decidual NK cells act as sentinel cells under circumstances of ongoing inflammation to inhibit the activation of autoimmune T cells, including TH17 cells. This directly or indirectly controls inflammation which is clearly critical for maintaining tolerance at the fetal–maternal interface. Interestingly, the differentiation of TH17 cells is regulated by circadian rhythms. The transcription factor, nuclear factor IL-3-regulated (NFIL3), can directly bind and repress the RORgt promoter, suppressing TH17 cell development. Through the transcription factor reverse erythroblastosis virus alpha, NFIL3 links TH17 cell development to the circadian network and regulates TH17 cell frequencies through the light cycle.77,78 In addition, NFIL3 is also essential for the development of NK cells, especially the formation of mature NK cells.79 Therefore, these findings are further evidence that NK cells are important in the regulation of TH17 cells. In pregnant Nfil3−/− mice, more decidual TH17 cells and increased fetal loss have been observed.70
Other lymphocytes are also involved in the regulation of TH17 cells. Wang et al.80 reported that human CD4+CD25+ Treg cells in healthy controls inhibited IL-17 secretion through cell-to-cell contact, and this regulation was disturbed in cases of unexplained RSA. In the joints of patients with rheumatoid arthritis, isolated Treg cells have also been shown to inhibit the secretion of IL-17 from effector T cells. Activated IL-10+ Treg cells required type Ι interferon signaling that depends on IL-12 family cytokines or the Toll-like receptor adapter protein myeloid differentiation primary response gene 88 (MYD88).81 However, contradictory results have been published regarding the capacity of Treg cells to control TH17 cells. One recent study in mouse showed that reduced induction of Treg cells does not cause substantial injury to the intestine and the proportion of TH17 cells remains unchanged.82 These data suggest that other cells may play a compensatory role in controlling TH17 cells. In addition to Foxp3+ Treg cells, mouse CD4+Foxp3− IL-10-producing type 1 Treg cells (Tr1) cells have also been shown to control TH17 in an IL-10-dependent manner.83
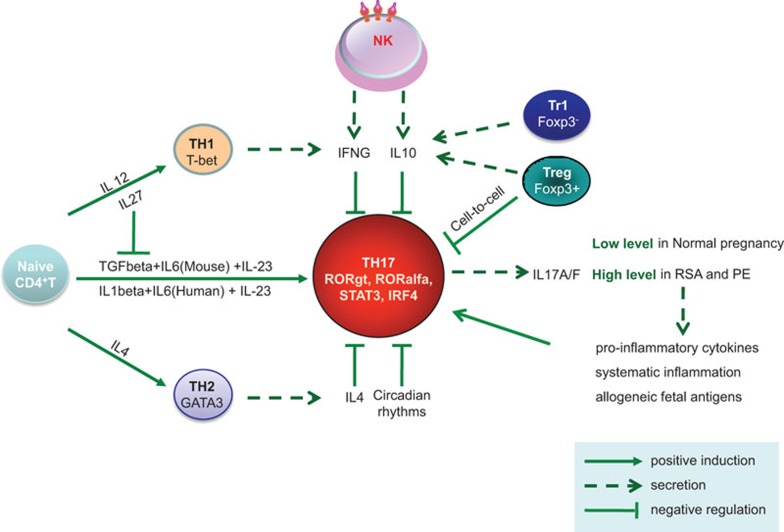
Understanding of the regulatory mechanisms was made more complex by the discovery that Treg cells can switch to a TH17-like phenotype when stimulated by allogeneic antigen-presenting cells, especially monocytes, in the presence of certain cytokines such as IL-2 and IL-15 and further enhanced by exogenous IL-1beta, IL-23 and IL-21,84 suggesting plasticity in the immune response and in immune cell phenotyping. This Treg-TH17 switch is suppressed by indoleamine-2,3-dioxygenase.85 Human decidual CD14+ monocytes86 and extravillous trophoblast cells have been found to express indoleamine-2,3-dioxygenase.87 Other cytokines, such IL-488 and IL-27,89 are also known to inhibit TH17 differentiation. IL-27 counters the polarization of naive CD4+ T cells, resulting in the inhibition of TH17 cell development.90 In contrast, IL-27 has little or no effect on mature TH17 cells.91 TGF-β is required for TH17 cell differentiation in mice, but it inhibits this polarization in humans50 (Figure 1).
Figure 1.
Key pathways of regulating TH17 cells during pregnancy. Naive CD4+ T helper cell develop into different T helper subsets, including TH1, TH2, TH17, Treg and Tr1 cells, under different cytokine environments. TH17 cells, characterized by producing IL-17, have an important role in pregnancy and have been upregulated in RSA and PE patients. Meanwhile, these TH17 cells and IL-17 cytokines are regulated under complex immune network. CD56brightCD16− decidual NK cells can inhibit inflammatory TH17 cells through IFNG and IL-10. Furthermore, IFNG from TH1, IL-4 from TH2, and IL-10 from Treg and Tr1 cells also have the ability to inhibit TH17 cells. IL-27 counters the polarization of naive CD4+ T cells, but has little or no effect on mature TH17 cells. TH17 cells are also affected by circadian rhythms. IFNG, interferon gamma; NK, natural killer; PE, pre-eclampsia; RSA, recurrent spontaneous abortion; TH17, T helper 17; Treg, T regulatory; Tr1, type 1 Treg cell.
Recent findings have also indicated that not all TH17 cells are pro-inflammatory. High Il23r expression is required for inflammatory function of pathogenic TH17 cells.53 In humans, pro-inflammatory TH17 cells stably express multidrug resistance type 1 and have a CCR6+CXCR3hiCCR4loCCR10−CD161+ phenotype.92 Additionally, it has been reported that TGF-β3 together with IL-6 drives the development of pathogenic TH17 cells compared with their non-pathogenic counterparts, which are induced by TGF-β1 and IL-6. These data suggest that TH17 subpopulations with diverse functions may exist.93 Furthermore, hyperactive TH17 cell increases susceptibility to inflammatory diseases. In pregnancy, adoptive transfer of TH17 cells polarized by cytokines IL-6 and TGF-β induces a higher fetal abortion rate.70 More investigations are needed to explore whether these pro-inflammatory TH17 or nonpathogenic TH17 subsets exist at the maternal-fetal interface and how the differential regulatory mechanisms manipulate these TH17 subsets.
Concluding remarks
TH17 cells were originally proposed as a new lineage of effector TH cells, the study of which has been one of most fascinating areas in immunology. To account for TH17 cell involvement, the former TH1/TH2 paradigm of pregnancy has been modified into TH1/TH2/TH17/Tr1 and Treg paradigms, each of which involves complicated interactions with decidual NK cells, dendritic cells, monocytes and extravillous trophoblast cells.94 In recent years, we have learned that TH17 cells produce an array of pro-inflammatory cytokines and host defense molecules and therefore, may play an important role in preventing pathological infection in pregnancy, although a defensive role for TH17 cells in reproductive organs has not yet been reported. More information is needed to clarify the role of TH17 cells in the establishment and defense mechanisms of normal pregnancy. We propose that the differentiation of TH17 cells is affected by inflammatory stimuli, including the local allogeneic fetal antigens, systematic inflammation and increased pro-inflammatory cytokines experienced during pregnancy.
In human recurrent pregnancy loss and PE, excessive TH17 cells numbers and high levels of IL-17, IL-6 and IL-1beta have been identified, indicating that uncontrolled TH17 cells may emerge as an important mediator of inflammation and tissue damage in the diseases of pregnancy. Understanding the regulation of TH17 cells might help us to design new therapeutic approaches to control these diseases that impact upon postnatal health of the mother and her child. For example, decidual NK cells, Treg cells and Tr1 cells have been shown to be capable of regulating TH17 cells through IFNG and/or IL-10. Inhibition specific for RORgt or IL-23/IL-23R blockade may also be attractive approaches to control TH17 cells and should be safe for the fetus.
However, numerous questions remain with respect to TH17 cells. For example: What antigens from an allogeneic fetus can affect TH17 cells and by what mechanism? What is the relationship between TH17 cells and other types of T cells? Can these cells interconvert from one lineage to another? What is the molecular control of this cross-lineage differentiation? Although we already have some knowledge regarding the differentiation of TH17 cells, how their effector functions are regulated is still poorly understood. In pregnancy disease models, do TH17 cells generate in situ or do they migrate into inflamed tissues? Further study is also needed on the function of TH17 cells in normal pregnancy and several diseases of pregnancy, including RSA and PE. Answering these questions will improve our understanding of how the fetal–maternal interface operates and may also lead to the development of new therapies.
Acknowledgments
We sincerely apologize to colleagues whose work could not be adequately discussed or cited due to the brevity of this review. Our work is supported by the Natural Science Foundation of China Grants 81202367 and 81330071.
The authors declare no competing financial interests.
References
- Dong C. T-H 17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Ouyang WJ, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Ito M, Shima T. Clinical implication of recent advances in our understanding of IL-17 and reproductive immunology. Expert Rev Clin Immunol. 2011;7:649–657. doi: 10.1586/eci.11.49. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443–448. doi: 10.1016/j.cytogfr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol. 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou GX, et al. Study on the relationship between Th17 cells and unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2011;65:503–511. doi: 10.1111/j.1600-0897.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Hao CF, Lin Y, Yin GJ, Bao SH, Qiu LH, et al. Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. J Reprod Immunol. 2010;84:164–170. doi: 10.1016/j.jri.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Toldi G, Rigo J, Stenczer B, Vasarhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2011;66:223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- Saito S. Th17 cells and regulatory T cells: new light on pathophysiology of preeclampsia. Immunol Cell Biol. 2010;88:615–617. doi: 10.1038/icb.2010.68. [DOI] [PubMed] [Google Scholar]
- Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon. Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Vince GS, Johnson PM. Is there a Th2 bias in human pregnancy. J Reprod Immunol. 1996;32:101–104. doi: 10.1016/s0165-0378(96)00995-3. [DOI] [PubMed] [Google Scholar]
- Raghupathy R. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin Immunol. 2001;13:219–227. doi: 10.1006/smim.2001.0316. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chen ZL, Smith GN, Croy BA. Natural killer cell-triggered vascular transformation: maternal care before birth. Cell Mol Immunol. 2011;8:1–11. doi: 10.1038/cmi.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy—an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ito M, Yoneda S, Shiozaki A, Hidaka T, Saito S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am J Reprod Immunol. 2010;63:104–109. doi: 10.1111/j.1600-0897.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009;183:7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- Ostojic S, Dubanchet S, Chaouat G, Abdelkarim M, Truyens C, Capron F. Demonstration of the presence of IL-16 IL-17 and IL-18 at the murine fetomaternal interface during murine pregnancy. Am J Reprod Immunol. 2003;49:101–112. doi: 10.1034/j.1600-0897.2003.01150.x. [DOI] [PubMed] [Google Scholar]
- Pongcharoen S, Niumsup P, Sanguansermsri D, Supalap K, Butkhamchot P. The effect of interleukin-17 on the proliferation and invasion of JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. 2006;55:291–300. doi: 10.1111/j.1600-0897.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Pongcharoen S, Supalap K. Interleukin-17 increased progesterone secretion by JEG-3 human choriocarcinoma cells. Am J Reprod Immunol. 2009;61:261–264. doi: 10.1111/j.1600-0897.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia EA, Chávez-Robles B, Sánchez-Hernández PE, Núñez-Atahualpa L, Martín-Máquez BT, Muñoz-Gómez A, et al. IL-17 increased in the third trimester in healthy women with term labor. Am J Reprod Immunol. 2011;65:99–103. doi: 10.1111/j.1600-0897.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- Heidt S, Segundo DS, Chadha R, Wood KJ. The impact of Th17 cells on transplant rejection and the induction of tolerance. Curr Opin Organ Transpl. 2010;15:456–461. doi: 10.1097/MOT.0b013e32833b9bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanidziar D, Koulmanda M. Inflammation and the balance of Treg and Th17 cells in transplant rejection and tolerance. Curr Opin Organ Transpl. 2010;15:411–415. doi: 10.1097/MOT.0b013e32833b7929. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347–353. doi: 10.1093/molehr/gah044. [DOI] [PubMed] [Google Scholar]
- Yang H, Qiu L, Chen G, Ye Z, Lü C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008;89:656–661. doi: 10.1016/j.fertnstert.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochanuna Z, Geiger-Maor A, Dembinsky-Vaknin A, Karussis D, Tykocinski ML, Rachmilewitz J. Inhibition of effector function but not T cell activation and increase in FoxP3 expression in T cells differentiated in the presence of PP14. PLoS ONE. 2010;5:e12868. doi: 10.1371/journal.pone.0012868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Jefferies M. Revised definition of pre-eclampsia. Lancet. 1988;1:809–812. doi: 10.1016/s0140-6736(88)91667-4. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leuk Biol. 2013;94:247–257. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211:165–179. doi: 10.1084/jem.20130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Hu YL, Wang ZQ, Zheng MM, Zhao X. Imbalance of T-cell transcription factors contributes to the Th1 type immunity predominant in pre-eclampsia. Am J Reprod Immunol. 2010;63:38–45. doi: 10.1111/j.1600-0897.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1: Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–555. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplänter C, Stadelmann C, Yu P, et al. The development of inflammatory T-H-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Sacks GP, Studena K, Sargent IL, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- Smarason AK, Gunnarsson A, Alfredsson JH, Valdimarsson H. Monocytosis and monocytic infiltration of decidua in early-pregnancy. J Clin Lab Immunol. 1986;21:1–5. [PubMed] [Google Scholar]
- Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol. 2003;131:490–497. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumandakis E, Koumandaki I, Kaklamani E, Sparos L, Aravantinos D, Trichopoulos D. Enhanced phagocytosis of mononuclear phagocytes in pregnancy. Br J Obstet Gynaecol. 1986;93:1150–1154. doi: 10.1111/j.1471-0528.1986.tb08636.x. [DOI] [PubMed] [Google Scholar]
- Austgulen R, Lien E, Liabakk NB, Jacobsen G, Arntzen KJ. Increased levels of cytokines and cytokine activity modifiers in normal-pregnancy. Eur J Obstetr Gynecol Reprod Biol. 1994;57:149–155. doi: 10.1016/0028-2243(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Melczer Z, Bánhidy F, Csömör S, Tóth P, Kovács M, Winkler G, et al. Influence of leptin and the TNF system on insulin resistance in pregnancy and their effect on anthropometric parameters of newborns. Acta Obstet Gyn Scan. 2003;82:432–438. [PubMed] [Google Scholar]
- Sacks GP, Seyani L, Lavery S, Trew G. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum Reprod. 2004;19:1025–1030. doi: 10.1093/humrep/deh179. [DOI] [PubMed] [Google Scholar]
- Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- Hahn S, Huppertz B, Holzgreve W. Fetal cells and cell free fetal nucleic acids in maternal blood: new tools to study abnormal placentation. Placenta. 2005;26:515–526. doi: 10.1016/j.placenta.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Rusterholz C, Huppertz B, Malek A, Schneider H, Holzgreve W, et al. A comparative study of the effect of three different syncytiotrophoblast micro-particles preparations on endothelial cells. Placenta. 2005;26:59–66. doi: 10.1016/j.placenta.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu BQ, Li X, Sun R, Tong X, Ling B, Tian Z, et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc Natl Acad Sci USA. 2013;110:E231–E240. doi: 10.1073/pnas.1206322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Shi S, Ljunggren HG, Cava AL, Van Kaer L, Shi FD, et al. NK cells inhibit T-bet-deficient, autoreactive Th17 cells. Scand J Immunol. 2012;76:559–566. doi: 10.1111/j.1365-3083.2012.02773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JW, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, et al. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–1921. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Fazekas G, Hara H, Tabira T. Mechanism of natural killer (NK) cell regulatory role in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;163:24–30. doi: 10.1016/j.jneuroim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang BN, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu BQ, Wang F, Sun R, Ling B, Tian Z, Wei H. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology. 2011;133:350–359. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342:727–730. doi: 10.1126/science.1243884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Seillet C, Huntington ND, Gangatirkar P, Axelsson E, Minnich M, Brady HJ, et al. Differential requirement for Nfil3 during NK cell development. J Immunol. 2014;192:2667–2675. doi: 10.4049/jimmunol.1302605. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Hao CF, Qu QL, Wang X, Qiu LH, Lin QD. The deregulation of regulatory T cells on interleukin-17-producing T helper cells in patients with unexplained early recurrent miscarriage. Hum Reprod. 2010;25:2591–2596. doi: 10.1093/humrep/deq198. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859–4874. doi: 10.1172/JCI65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–8120. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca P, Cantoni C, Vitale M, Prato C, Canegallo F, Fenoglio D, et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc Natl Acad Sci USA. 2010;107:11918–11923. doi: 10.1073/pnas.1001749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig A, Rieger L, Kapp M, Sütterlin M, Dietl J, Kämmerer U. Indoleamine 2,3-dioxygenase (IDO) expression in invasive extravillous trophoblast supports role of the enzyme for materno-fetal tolerance. J Reprod Immunol. 2014;61:79–86. doi: 10.1016/j.jri.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Cooney LA, Towery K, Endres J, Fox DA. Sensitivity and resistance to regulation by IL-4 during Th17 maturation. J Immunol. 2011;187:4440–4450. doi: 10.4049/jimmunol.1002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, et al. IL-27 Blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu BQ, Tian ZG, Wei HM. Subsets of human natural killer cells and their regulatory effects. Immunology. 2014;141:483–489. doi: 10.1111/imm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]