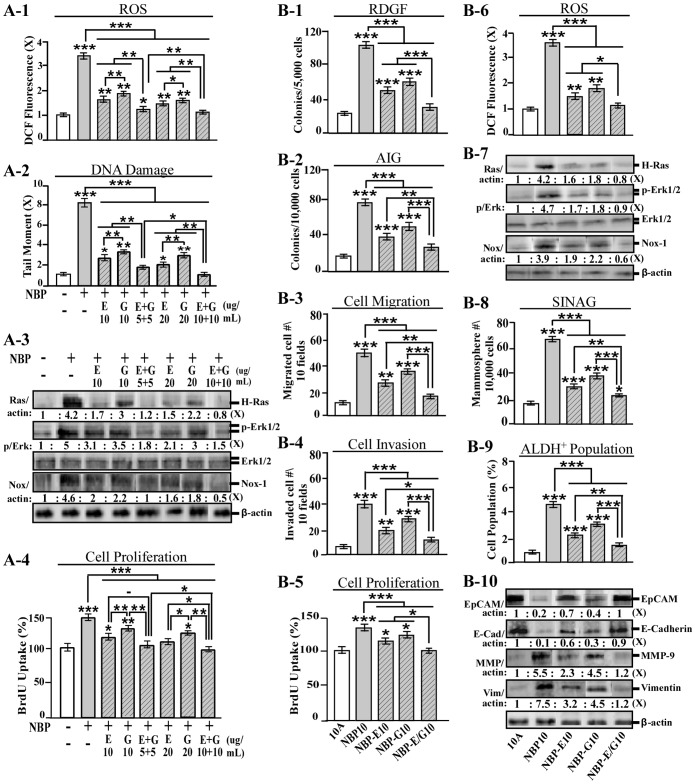
Figure 4. Intervention of NBP-induced carcinogenesis.
(A-1 to A-4) MCF10A cells were treated with NBP in the absence and presence of ECG (E), EGCG (G), or a combination of ECG and EGCG (E+G) for 24 h. (B-1 to B-10) MCF10A (10A) cells were exposed to NBP in the absence and presence of 20 µg/mL ECG, 20 µg/mL EGCG, or combined 10 µg/mL ECG and 10 µg/mL EGCG (E/G) for 10 cycles, resulting in the NBP10, NBP-E10, NBP-G10, and NBP-E/G10 cell lines, respectively. (A-1 and B-6) Relative level of ROS as fold induction (X, arbitrary unit) was normalized by the level determined in untreated cells, set as 1. (A-2) Relative DNA damage was measured by a comet assay and normalized by the value of average tail moment determined in untreated counterpart cells, set as 1 (X, arbitrary unit). (A-3 and B-7) Cell lysates were analyzed by immunoblotting using specific antibodies to detect levels of H-Ras, phosphorylated-Erk1/2 (p-Erk1/2), Erk1/2, and Nox-1, with β-actin as a control, and these levels were quantified by densitometry. Levels of H-Ras and Nox-1 were calculated by normalizing with the level of β-actin and the level set in untreated control cells as 1 (X, arbitrary unit). Levels of specific phosphorylation of Erk1/2 (p/Erk) were calculated by normalizing the levels of p-Erk1/2 with the levels of Erk1/2, then the level set in control cells as 1 (X, arbitrary unit). (A-4 and B-5) Relative cell proliferation was determined and normalized by the value of BrdU detected in untreated cells, set as 100%. (B-1) To determine cellular acquisition of RDGF, cells were maintained in LM medium for 10 days. Cell colonies ≥0.5 mm diameter were counted. (B-2) To determine cellular acquisition of AIG, cells were seeded in soft agar for 14 days. Cell colonies ≥0.1 mm diameter were counted. (B-3) Cellular migratory and (B-4) invasive activities were determined by counting the numbers of cells translocated through a polycarbonate filter without or with coated Matrigel, respectively, in 10 arbitrary visual fields. (B-8) To determine cellular acquisition of the ability of serum-independent non-adherent growth (SINAG), cells were seeded in non-adherent cultures for 10 days; then, mammospheres (≥0.1 mm diameter) were counted. (B-9) Mammospheres were collected and trypsinized, and ALDH-expressing (ALDH+) cell population (%) was measured by flow cytometry. (B-10) Cell lysates were analyzed by immunoblotting using specific antibodies to detect levels of EpCAM, E-cadherin, MMP-9 and Vimentin, with β-actin as a control, and these levels were quantified by densitometry. The levels of EpCAM, E-cadherin, MMP-9 and Vimentin were calculated by normalizing with the level of β-actin and the level set in untreated control cells as 1 (X, arbitrary unit). Columns, mean of triplicates; bars, SD. All results are representative of three independent experiments. Statistical significance is indicated by * P<0.05, ** P<0.01, and *** P<0.001.