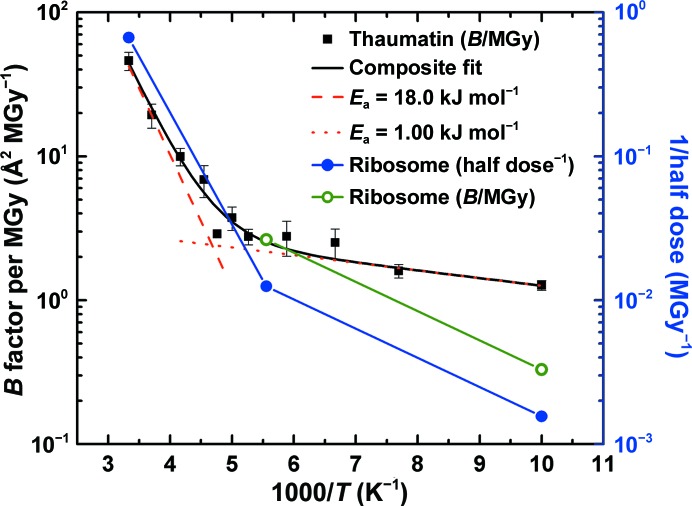
Figure 3.
Half dose (solid blue circles, right scale) and B-factor sensitivity (open green circles, left scale) data for 70S ribosome crystals from the present work compared with B-factor sensitivity data for thaumatin crystals from Warkentin & Thorne (2010 ▶) (solid black squares, left scale). Between 180 and 300 K, the temperature dependence of the ribosome half dose roughly agrees with that of the thaumatin B-factor sensitivities. At lower temperatures, the ribosome data appear to be more strongly temperature dependent. This may result because solvent, free-radical and protein conformational mobility may persist to lower temperatures in ribosome crystals owing to the much larger solvent channels, moving the ‘kink’ separating the high-temperature and low-temperature regimes evident in the thaumatin data to lower temperatures.