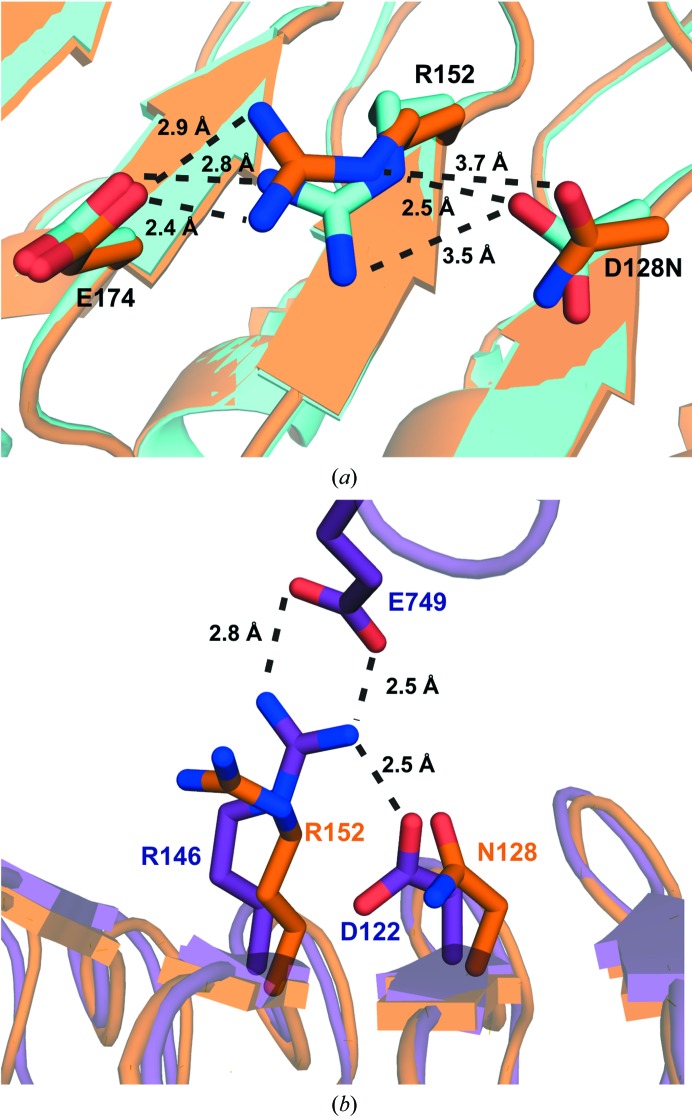
Figure 4.
(a) Overlay of wild-type OsSERK2 (cyan) and OsSERK2 D128N (orange). In the wild-type protein, Asp128 forms a salt bridge to Arg152. The D128N mutant breaks this interaction. Instead, Arg152 of the OsSERK2 D128N mutant forms a salt bridge with Glu174. (b) Overlay of OsSERK D128N (orange) and the BRI1–BAK1 complex (PDB entry 4lsx; purple). The analogous arginine in BAK1 (Arg146) forms a salt bridge with Glu749 from BRI1. BAK1 Asp122 forms a salt bridge with BAK1 Arg146 and helps to position BAK1 Arg146 for binding to BRI1. Our structure shows that OsSERK2 D128N is no longer able to make the analogous interaction, therefore leading to altered binding to BRI1 and the elg phenotype.