Abstract
There are two distinct modes of self-focus: analytical self-focus is abstract, general and evaluative whereas experiential self-focus is concrete, specific and non-evaluative. Using functional magnetic resonance imaging (fMRI), we investigated the neural bases of these two modes of self-focus in relation with brooding, the maladaptive form of rumination. Forty-one French-speaking right-handed healthy young adults (10 men, mean age ± s.d.: 21.8 ± 2.3 years) engaged in analytical and experiential self-focus triggered by verbal stimuli during fMRI. Brooding was measured with the 22-item Rumination Response Style scale. Individuals with lower brooding scores showed greater activation of the posterior cingulate cortex/precuneus during analytical than experiential self-focus, whereas individuals with higher brooding scores did not. This is consistent with the hypothesis that brooding is associated with less control over the nature of the self-focus engaged. These findings may help to refine our understanding of how rumination promotes depression through maladaptive self-focus.
Keywords: rumination, self-focus, self-reference, posterior cingulate cortex, fMRI
INTRODUCTION
Major depression is associated with an increased attention to the self, namely self-focus (Mor and Winquist, 2002). Self-focus may be operationalized in social neuroscience as the process by which individuals engage in self-referential processing (i.e. the appraisal of stimuli as strongly related to one’s own person) (Lemogne et al., 2012). Self-referential processing in healthy subjects is associated with activation of the cortical midline structures (CMS) (Fossati et al., 2003; Northoff et al., 2006). Several studies have examined the neural bases of self-referential processing in depressed individuals (Grimm et al., 2009; Lemogne et al., 2009; Johnson et al., 2009; Yoshimura et al., 2010) or vulnerable individuals (Lemogne et al., 2011) and found either an increased or a decreased activation of the CMS. Although these discrepancies may result from methodological differences (Lemogne et al., 2012; Nejad et al., 2013), they may also result from the fact that self-focus is not a unitary process but rather encompasses at least two components, referred to as ‘analytical’ and ‘experiential’, respectively. Analytical self-focus is characterized by an abstract evaluation of the self as a continuous object of knowledge, whereas experiential self-focus is characterized by a concrete, awareness of the self as the subject of immediate experience in the moment (Watkins, 2008). Farb et al. (2007) found that anterior and posterior CMS to be more active during analytical than experiential self-focus, whereas left-lateralized regions, including dorsal and ventral lateral prefrontal cortex (PFC) and posterior parietal cortex, were more active during experiential than analytical self-focus. This study aimed to examine neural activations during analytical vs experiential self-focus among healthy subjects, in relation to individual differences in rumination.
According to Nolen-Hoeksema (1991), rumination is a style of response to psychological distress that involves repetitively and passively focusing on symptoms of distress and possible causes and consequences of theses symptoms. An exploratory factorial analysis of the Ruminative Responses Scale (RRS) yielded two factors labelled as reflective pondering (a purposeful turning inward to engage in cognitive problem solving to alleviate one’s depressive symptoms) and brooding (a passive comparison of one’s current situation with some unachieved standard) (Treynor et al., 2003). In contrast with reflective pondering, brooding is associated with negative affect and impaired cognitive control in healthy subjects, and with negative attentional biases, severity and duration of depressive episodes, and increased risk of relapse in currently and formerly depressed patients (Joormann et al., 2006; Whitmer and Banich, 2007; Nolen-Hoeksema et al., 2008). Several studies have examined the correlations between inter-individual differences in rumination and neural activations during functional magnetic resonance imaging (fMRI) tasks among healthy and depressed individuals (Siegle et al., 2002; Ray et al., 2005; Johnson et al., 2009; Berman et al., 2011a,b; Farb et al., 2011; Hamilton et al., 2011; Vanderhasselt et al., 2011; Zhu et al., 2012; Paul et al., (2013); Nejad et al., 2013). Altogether, these studies suggest that (i) rumination scores are positively correlated with functional connectivity within the so-called ‘default mode network’ at rest (including the anterior and posterior midline structures); (ii) rumination scores are positively correlated with an increased activation of the lateral PFC during the processing of negative stimuli or externally oriented tasks, possibly underlying greater cognitive efforts to disengage from self-focus; and (iii) these differences are specifically associated with brooding but not reflective pondering. To our knowledge, only one fMRI study examined the neural bases of self-referential processing in relation with rumination among depressed and healthy individuals. Higher rumination scores were associated with greater anterior and posterior medial cortex activation during non-self-referential task, consistent with difficulties to disengage from spontaneous self-focus (Johnson et al., 2009). To our knowledge, however, the neural correlates of rumination have never been examined in relation to analytical vs experiential self-focus among healthy subjects.
To address this question, we conducted a method in two steps: (i) we performed a contrast analysis (analytical vs experiential self-focus) to determine regions more specifically activated during one condition compared with the other and (ii) we performed correlation analyses between rumination scores and the activity of regions identified in the first step during analytical vs experiential self-focus. Farb et al., (2007) and Johnson et al. (2009) found less activity in anterior and posterior CMS during experiential than analytical self-focus in healthy participants. First, we expected this difference of activity to be reduced in participants with high levels of rumination. In other words, we hypothesized that rumination would be associated with unwanted analytical self-focus during the experimental induction of experiential self-focus, thus leading to a negative correlation between rumination scores and activation of the anterior and posterior CMS, in analytical vs experiential self-focus. Such an outcome would be consistent with the idea that individuals with high levels of rumination are less able to control the nature of the type of self-focus they engage. Second, we thus hypothesized that rumination would be associated with more cognitive control during experiential self-focus in order to inhibit unwanted and automatic analytical self-focus, thus leading to a positive correlation between rumination scores and activation of the cognitive control network in experiential vs analytical self-focus. Third, we predicted that these correlations would be specific of the brooding component of rumination, thus highlighting the neural correlates of the dark side of self-focus.
MATERIALS AND METHODS
Participants
All participants were French-speaking right-handed healthy young adults and gave written informed consent after complete description of the study. The Ethics Committee for Biomedical Research of the Pitié-Salpêtrière Hospital approved the study. The volunteers were screened for past and present DSM-IV diagnoses with the Mini International Neuropsychiatric Interview (Sheehan et al., 1998) and for nicotine dependence with the Fagerström Scale (Heatherton et al., 1991). Exclusion criteria were current or past psychiatric disorders (including substance-related disorders), medical disorders or medication likely to affect cognition, left-handedness and prior meditation or psychotherapy experience. Forty-one right-handed healthy subjects were included in the study (10 men, 31 women, mean age ± s.d.: 21.8 ± 2.3 years). Vision was normal or corrected to near normal using contact lenses.
Procedure
The experiment took place over two sessions separated by an average interval (±s.d.) of 12.8 ± 8.3 days. The first session included in the following order: screening for exclusion criteria, questionnaires administration, training in differentiating analytical and experiential self-focus. At the second session, participants performed the fMRI task.
Questionnaires
Rumination was assessed with the French translation of the 22-item RRS (Nolen-Hoeksema and Morrow, 1993; Guimpel et al., 2012). Participants are asked to indicate how often they engage in each of 22 ruminative thoughts or behaviours when they feel sad, blue or depressed. Separator scores were computed for Brooding (RRSb) and Reflective pondering (RRSr) subscales. Both subscales have been found to have acceptable internal consistency (α = 0.77 and 0.72 for RRSb and RRSr, respectively) and test–retest reliability (r = 0.62 and 0.60 for RRSb and RRSr, respectively). Depressive symptoms over the preceding two weeks were measured with the Beck Depression Inventory II (BDI-II), which is a reliable and valid questionnaire of 21 items (Bourque and Beaudette, 1982).
Self-focus task
Participants were trained as regards the distinction between experiential and analytical self-focus. The stimuli were 28 short phrases designating sensations, feelings or thoughts selected to trigger self-focused attention (e.g. ‘the physical sensations in your body’) adapted from Nolen-Hoeksema and Morrow’s (1993) rumination task. The instructions were adapted from Watkins and Teasdale (2001) and both conditions (i.e. analytical or experiential self-focus) used the same stimuli. The instructions in the experiential self-focus were: ‘As you read the items, use your imagination and concentration to focus your mind on each experience. Spend a few moments visualizing and concentrating on your experience, attempting to find a phrase, image or set of words that best describes the quality of what you sense’. The instructions in the analytical induction were: ‘As you read the items, use your imagination and concentration to think about the causes, meanings and consequences of the items. Spend a few moments visualizing and concentrating on each item, attempting to make sense of and understand the issues raised by each item’. The participants worked at their own pace for 10 min in each self-focus condition. The order of the conditions was counterbalanced across participants.
fMRI task design
A list of 36 short phrases (6 for the practice run and 30 for the scanning runs) describing sensations, feelings and thoughts were constructed, adapted from Nolen-Hoeksema and Morrow’s (1993) rumination task (please see Supplementary Appendix S1 for the list of stimuli). These items had neutral emotional valence and were constructed to trigger self-referential processing. Each item was used two times: one time in analytical self-focus condition and one time in experiential self-focus condition. The order in which stimuli were used was randomized for each subject. Immediately before the task, the analytical and experiential instructions of self-focus instructions were recalled to the participants. Each participant completed one practice run outside the scanner and four scanning runs. Each run consisted of two blocks of analytical self-focus and two blocks of experiential self-focus, in random order. Before each block, an instruction cue was presented for 8 s: ‘Think about …’ for the analytical self-focus condition and ‘Focus your attention on your experience of …’ for the experiential self-focus condition. During each block, participants performed analytical or experiential self-focus during 30 s based on three items presented during 10 s each (the stimulus presented for 2.5 s and a fixation cross presented for 7.5 s). After each block, an instruction to disengage from self-focus was presented for 3 s, followed by a rest fixation cross presented for 7.5 s. Owing to technical problems, fMRI data were lost regarding one run for four participants and two runs for one participant.
fMRI scanning
Stimuli were generated and presented with E-Prime 2.0 and projected on a plexiglas screen mounted at the end of the scanner bore. Five functional runs of 102 contiguous volumes were acquired on a 3T Trio TIM MR-scanner (Siemens Medical Solutions, Erlangen, Germany) with Siemens standard 12-channel head coil. Subjects’ head movements were restrained by foam paddings, inside of the head coil. Functional images covering the whole brain were acquired using a T2-weighted gradient echo, echo planar imaging (EPI) sequence, sensitive to blood oxygen level-dependent signal, employing the following parameters: 41 axial slices, repetition time: 2 s, echo time: 25 ms, flip angle: 90°, bandwidth: 2230 Hz, matrix: 64 × 64, field of view: 192 × 192 mm2, isotropic voxel size of 3 × 3 × 3 mm3, GRAPPA acceleration factor: 2. Each run lasted 194 s. The first two volumes of each run were discarded to reach signal equilibrium. High-resolution three-dimensional T1 weighted images (3D fast gradient echo inversion recovery sequence, inversion time: 400 ms, repetition time: 2300 ms, echo time: 4.18 ms, bandwidth: 150 Hz, flip angle: 98°, matrix: 256 × 256, field of view: 220 × 220 mm2, voxel size: 1 × 1 × 1 mm3) were acquired for anatomical localization.
fMRI data analysis
We used SPM5 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) for data analysis. EPI volumes were corrected for slice timing, realigned to the first image, co-registered with the high-resolution T1-weighted image, and normalized into a standard stereotactic space. The normalization used the Montreal Neurological Institute (MNI) template and the transformations computed during the segmentation of the high-resolution T1-weighted image. Finally, the normalized EPI volumes were smoothed using an isotropic Gaussian kernel filter of 8 mm full-width at half-maximum. Each trial onset was convolved with the canonical haemodynamic response function (HRF) scaled relative to the self-focus condition block and rest fixation cross durations (30 s and 7.5 s, respectively), to create regressors of interest. A high-pass filter with a default cutoff of 128 s was applied and the motion realignment parameters were included as regressors of non-interest. The analytical vs experiential first-level individual contrast images were obtained for the HRF estimates.
Statistical analysis
First, we aimed to identify the specific neural correlates of the two modes of self-focus. We performed two random effect second-level one-sample t-tests with the analytical vs experiential first-level contrast images. A whole-brain analysis was performed using a combined threshold of uncorrected voxel P < 0.005 with 20 contiguous voxels, to produce a reasonable balance between Type I and Type II error rates (Lieberman and Cunningham, 2009).
Second, these results were used as inclusive masks in further analyses to examine how the activation of these regions was modulated by levels of rumination. We conducted multiple regression analyses using analytical vs experiential contrast images as dependent variables and RRSb scores and RRSr scores separately as independent variables. These analyses were threshold at uncorrected voxel P < 0.005 with 20 contiguous voxels (Lieberman and Cunningham, 2009). Whenever a significant correlation was found in one region, we conducted post hoc correlation analyses in SPSS (SPSS Inc, Chicago, IL) between rumination scores and mean activation (extracted with MarsBaR toolbox) in the analytical vs experiential contrast.
RESULTS
Psychometric results
RRSb and RRSr scores means (±s.d.) were, respectively, 9.2 ± 3.3 and 9.2 ± 3.4. As expected, participants had low BDI-II scores (mean ± s.d.: 2.8 ± 3.3). Depressive symptoms were significantly and positively correlated with RRSb (r = 0.442, P = 0.004) but not with RRSr (r = 0.199, P = 0.212).
fMRI results
Table 1 displays regions that were more active during analytical than experiential self-focus, and inversely. As regards analytical vs experiential self-focus, the largest cluster was located in the posterior CMS and encompassed bilateral posterior cingulate cortices, precuneus and cuneus (Brodmann areas: 31, 23 and 18) (Figure 1, left panel). As regards experiential vs analytical self-focus, the largest clusters were found in lateral cortical structures only: bilateral middle and inferior frontal gyrus, bilateral inferior parietal lobule, bilateral lateral temporal cortex and right fronto-insular cortex (Figure 1, right panel).
Table 1.
Regions that were more active during analytical than experiential self-focus and inversely, in a whole-brain one-sample t-test
| Regions | Brodmann area | MNI coordinates (x, y, z) | t | Cluster corrected P | Cluster size (voxels) |
|---|---|---|---|---|---|
| Analytical > experiential | |||||
| Posterior cingulate, precuneus, cuneus and R cerebellum posterior lobe declive | 31, 23, 18 | 2, −58, 24 | 5.90 | <0.001 | 6322 |
| 8, −56, 12 | |||||
| −10, −76, 14 | |||||
| 20, −68, −20 | |||||
| L ventromedial frontal gyrus | 11 | −10, 54, −10 | 4.10 | 0.844 | 90 |
| L superior frontal gyrus | 6 | −4, 6, 64 | 4.88 | 0.718 | 106 |
| 9 | −14, 38, 40 | 3.35 | 1.000 | 20 | |
| 9 | −16, 54, 34 | 3.26 | 0.997 | 48 | |
| Superior temporal gyrus | 38 | 56, 12, −14 | 4.01 | 0.997 | 48 |
| −50, 14, −24 | 3.71 | 0.995 | 51 | ||
| Middle temporal gyrus | 21 | −56, −8, −10 | 3.47 | 1.000 | 33 |
| −46, −36, −2 | 3.41 | 0.998 | 45 | ||
| 46, −30, 0 | 3.35 | 0.998 | 46 | ||
| Experiential > analytical | |||||
| L middle and inferior frontal gyrus | 46, 10 | −44, 46, 18 | 6.10 | <0.001 | 673 |
| R middle and inferior frontal gyrus | 46 | 16, 34, −16 | 4.84 | 0.001 | 547 |
| R fronto-insular cortex | 44, 6, 13 | 58, 2, 12 | 5.35 | <0.001 | 634 |
| 48, 0, 8 | |||||
| Inferior parietal lobule | 40 | −58, −40, 46 | 5.33 | <0.001 | 1452 |
| 50, −36, 48 | 5.54 | <0.001 | 1271 | ||
| R fusiform and middle temporal gyrus | 37, 21 | 50, −52, −18 | 4.35 | <0.001 | 653 |
| L fusiform and inferior temporal gyrus | 37, 19 | −42, −32, −18 | 4.95 | 0.002 | 501 |
| L temporo-occipital junction cortex | 19 | −28, −70, 26 | 4.69 | 0.372 | 152 |
| L middle occipital gyrus | 18 | −34, −78, 0 | 3.74 | 0.998 | 46 |
| L superior temporal gyrus | 22 | −52, −8, 2 | 3.42 | 1.000 | 24 |
| L superior parietal lobule | 7 | −16, −62, 60 | 3.76 | 0.271 | 171 |
| Medial frontal gyrus supplementary motor area | 6 | 6, −8, 62 | 4.43 | 0.249 | 176 |
| R middle frontal gyrus | 8 | 42, 32, 42 | 3.55 | 0.999 | 41 |
| 10 | 42, 44, 24 | 3.30 | 0.958 | 69 | |
| R inferior frontal gyrus | 10 | 34, 62, 4 | 3.38 | 1.000 | 24 |
| L insula | 13 | −32, −18, 8 | 3.65 | 1.000 | 34 |
| −36, −6, 12 | 3.30 | 1.000 | 23 | ||
| R parahippocampal gyrus | 28 | 30, −14, −20 | 3.24 | 0.990 | 56 |
L, left; R, right. MNI coordinates of maximum t-scores are shown for each cluster. P < 0.005 uncorrected voxel threshold, and 20 voxels extend threshold. Clusters corrected P ≤ 0.001 in bold.
Fig. 1.
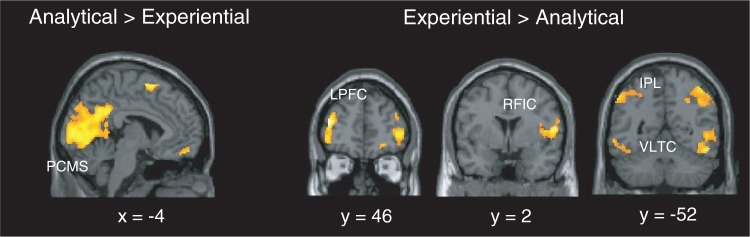
Regions specifically activated during one self-focus condition compared with the other in a whole-brain one-sample t-test. (20 contiguous voxels, P < 0.005) The largest areas activated in analytical vs experiential contrast are displayed on the left panel, and those activated in experiential vs analytical contrast are displayed on the right panel. PCMS, posterior cortical midline structures; LPFC, lateral prefrontal cortex; RFIC, right fronto-insular cortex; IPL, inferior parietal lobule; VLTC, ventrolateral temporal cortex.
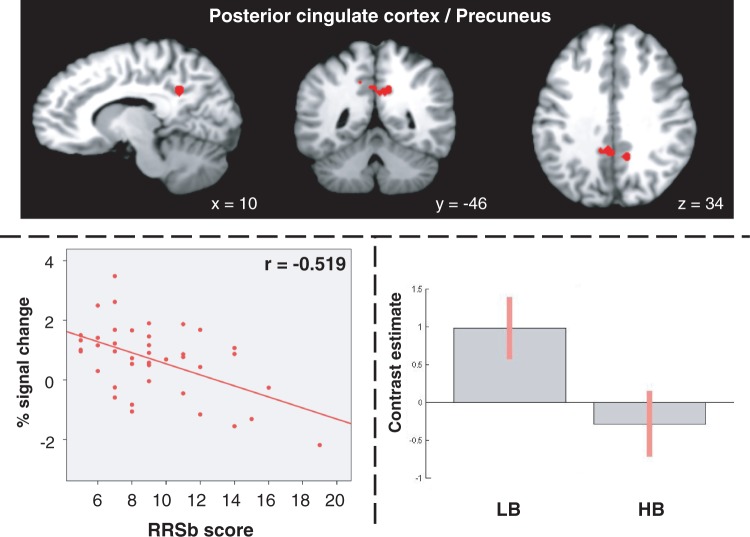
Among these regions of interest (ROIs), Table 2 displays those in which the differential activation between the two modes of self-focus was associated with RRSb scores. The multiple regression analysis revealed negative correlation between RRSb scores and neural activity during analytical vs experiential self-focus in the posterior cluster that was more specifically activated during analytical vs experiential self-focus (r = −0.519, P = 0.001), and specifically in the posterior cingulate cortex/precuneus (Figure 2).
Table 2.
Negative correlation between RRSb scores and ROIs’ activation during analytical vs experiential self-focus in multiple regression analyses
| Regions | MNI coordinates (x, y, z) | t | Cluster size (voxels) |
|---|---|---|---|
| Posterior cingulate cortex/precuneus | 10, −46, 34 | 4.04 | 71 |
| −12, −40, 36 | 3.65 | ||
| −2, −42, 36 | 3.34 |
MNI coordinates of maximum t-scores are shown for each cluster. Voxel uncorrected threshold: P < 0.005 and extend threshold: 20 voxels.
Fig. 2.
Images (top panel) and scatter plot (bottom left panel) show the posterior cingulate/precuneus region whose activation during analytical vs experiential self-focus is negatively correlated with the RRSb score (20 contiguous voxels, P < 0.005). (bottom right panel) Bar graphs show the magnitude of activations associated with the analytical vs experiential contrast in the low brooding (LB) and high brooding (HB) groups, based on a RRSb score median-split.
Scatter plots between mean percent signal change of the significant clusters and RRSb scores revealed an outlier with a high RRSb score of 19 (Figure 2). The analysis was repeated after removing this outlier and yielded similar results. No correlation was found between RRSr scores and the activity of any selected brain region during analytical vs experiential self-focus condition. After adjustment for BDI-II scores, this correlation remained significant.
DISCUSSION
The aim of this study was to examine the association between rumination and the neural activations during analytical vs experiential self-focus in healthy subjects. Consistent with our first hypothesis, healthy subjects with lower brooding scores showed greater activation of the posterior CMS during analytical than experiential self-focus, whereas those with higher brooding scores showed less difference. In contrast, our second hypothesis was not supported as we did not find any correlation between rumination scores and activation of the cognitive control network in experiential vs analytical self-focus. Finally, consistent with our third hypothesis, our results were specific to brooding and we did not find any correlation between reflective pondering score and neural activation during analytical vs experiential self-focus.
The results of the preliminary contrast analyses, which examined specific activations associated with analytical and experiential self-focus relative to each other, revealed similar results to Farb et al. (2007) and Johnson et al. (2009). Analytical self-focus specifically engaged a large cluster with the posterior CMS encompassing the posterior cingulate cortices, cuneus and precuneus. These regions are involved in mental imagery, autobiographical memory, self-projection, scene construction and theory of mind (Spreng et al., 2009). These functions are consistent with the nature of analytical self-focus which deals with the extended, narrative self rather than with immediate experience (Gallagher, 2000; Trope and Liberman, 2003). Relative to analytical self-focus, experiential self-focus engaged the bilateral dorsolateral prefrontal and inferior parietal lobules, both being part of the frontoparietal attention and control network (Corbetta et al., 2008; Vincent et al., 2008). These networks are, respectively, involved in attention reorienting and rapid adaptive control, consistent with the nature of experiential self-focus that requires directing and maintaining attention towards immediate experience. Furthermore, activations in the inferior parietal lobule (embodied self), the right mid-insula (interoceptive awareness) and the ventrolateral temporal (semantic system associated with concrete concepts) are also consistent with attention to the current, concrete experience of the self (Martin and Chao, 2001; Craig, 2010; Blanke, 2012).
Overall, the fact that individuals with higher brooding scores showed a reduced difference in posterior cingulate cortex/precuneus activity during analytical vs experiential self-focus suggests that they have more difficulties in differentiating the two conditions (i.e. they were less able to control the nature of the self-focus they engage).
Posterior CMS are known to show higher activity during stimulus-independent and task-unrelated processing compared with externally oriented tasks (Mason et al., 2007; Buckner et al., 2008). Recent studies found this region to display greater activity during externally oriented tasks and greater functional connectivity at rest in individuals prone to ruminate. For instance, Grimm et al. (2009) found higher activation of the precuneus during an externally oriented task in depressed individuals. Berman et al. (2011b) found greater functional connectivity between anterior and posterior CMS at rest in depressed individuals, and especially in those with higher brooding, but not reflection pondering scores. Closer to the present results, Johnson et al. (2009) found higher activation of a very similar region within the posterior CMS (Talairach coordinates of the peak: −4, −55, 32) during a concrete, internally oriented (but non-self-referential) task in depressed vs healthy individuals, especially in those with higher rumination scores. Interestingly, this structure was less modulated between analytical and experiential self-focus in the depressed subjects of Johnson et al. (2009) compared with healthy individuals, mirroring the present results obtained comparing high vs low brooders healthy subjects (Table 2, Figure 2). Therefore, negative correlations between brooding score and the posterior CMS during analytical vs experiential self-focus could be interpreted as a difficulty for brooders to disengage from spontaneous and unwanted thought during experiential vs analytical self-referential processing.
A limitation of the present design is that the neural results could be attributed to processes in general that have abstract vs concrete properties as opposed to these properties in the context, specifically, of self-focus. Contrasting with our results, meta-analyses showed a greater activation of the posterior cingulate cortex/precuneus during concrete vs abstract semantic processing of words, possibly explained by the role of these regions in mental imagery processes (Binder et al., 2009; Wang et al., 2010). However, recent studies showed a greater activation of these regions during abstract vs concrete mindset processing (Spunt et al., 2010; Gilead et al., 2013). To address this issue, further studies would need to add another level to the experimental paradigm involving abstract vs concrete processing in a non-self-focused context.
Some others limitations should be acknowledged. We did not use online or post-scanning subjective measures of automatic and unwanted thought during fMRI. We chose this method because we did not want to introduce another cognitively demanding task that would have interfered with automatic self-focus. Second, we only used neutral stimuli so that we could not examine the potential effects of stimuli emotional valence. Third, we excluded subjects with current or past depressive disorders. We chose this method to examine the neural correlates of rumination independently from depressive mood. However, rumination and analytical self-focus have been found to have detrimental effects in dysphoric or depressed subjects, specifically (Nolen-Hoeksema et al., 2008; Watkins, 2008). Further studies may explore the neural correlates of analytical vs experiential self-focus in relation to both rumination levels and current state of mood.
Overall, these limitations do not invalidate our main findings, showing that the maladaptive form of rumination, captured by a high brooding score, is associated with a reduced difference of the posterior cingulate cortex/precuneus activity between analytical and experiential self-focus. These findings may help to refine our understanding of how rumination paves the way to depression at a brain level.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
FUNDING
M.F. was supported by fundings from the Fondation pour la Recherche Médicale. P.F. was supported by a NARSAD Young Investigator Award 2003.
Conflict of Interest
None declared.
Supplementary Material
REFERENCES
- Berman MG, Nee DE, Casement M, et al. Neural and behavioral effects of interference resolution in depression and rumination. Cognitive, Affective & Behavioral Neuroscience. 2011a;11:85–96. doi: 10.3758/s13415-010-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive and Affective Neuroscience. 2011b;6:548–55. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nature Reviews: Neuroscience. 2012;13:556–71. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- Bourque P, Beaudette D. Étude psychometrique du questionnaire de dépression de Beck auprès d'un échantillon d'étudiants universitaires francophones. Canadian Journal of Behavioural Science/Revue Canadienne des Sciences du Comportement. 1982;14:211. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Structure & Function. 2010;214:563–77. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biological Psychiatry. 2011;70:366–72. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Gallagher II. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gilead M, Liberman N, Maril A. From mind to matter: neural correlates of abstract and concrete mindsets. Social Cognitive and Affective Neuroscience. 2014;9(5):638–45. doi: 10.1093/scan/nst031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimpel B, Douilliez C, Philippot P. French validation of brooding and reflection dimensions of the Ruminative Responses Scale (RRS). Annual meeting of the 41èmes Journées Scientifiques de Thérapie Comportementale et Cognitive. Paris, France: 2012, December. [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry. 2011;70:327–33. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37:269–80. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders. 2012;136:e1–11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Bergouignan L, Pelissolo A, Lehericy S, Fossati P. Negative affectivity, self-referential processing and the cortical midline structures. Social Cognitive and Affective Neuroscience. 2011;6:426–33. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4:305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–28. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N, Winquist J. Self-focused attention and negative affect: a meta-analysis. Psychological Bulletin. 2002;128:638–62. doi: 10.1037/0033-2909.128.4.638. [DOI] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–82. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cognition & Emotion. 1993;7:561–70. [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–24. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social Cognitive and Affective Neuroscience. 2013;8:56–64. doi: 10.1093/scan/nss070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective & Behavioral Neuroscience. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Falk EB, Lieberman MD. Dissociable neural systems support retrieval of how and why action knowledge. Psychological Science. 2010;21:1593–8. doi: 10.1177/0956797610386618. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research. 2003;27:247–59. [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychological Review. 2003;110:403–21. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, Kuhn S, De Raedt R. Healthy brooders employ more attentional resources when disengaging from the negative: an event-related fMRI study. Cognitive, Affective & Behavioral Neuroscience. 2011;11:207–16. doi: 10.3758/s13415-011-0022-5. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Human Brain Mapping. 2010;31:1459–68. doi: 10.1002/hbm.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134:163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E, Teasdale JD. Rumination and overgeneral memory in depression: effects of self-focus and analytic thinking. Journal of Abnormal Psychology. 2001;110:353–7. doi: 10.1037/0021-843x.110.2.333. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Banich MT. Inhibition versus switching deficits in different forms of rumination. Psychological Science. 2007;18:546–53. doi: 10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders. 2010;122:76–85. doi: 10.1016/j.jad.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry. 2012;71:611–7. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.