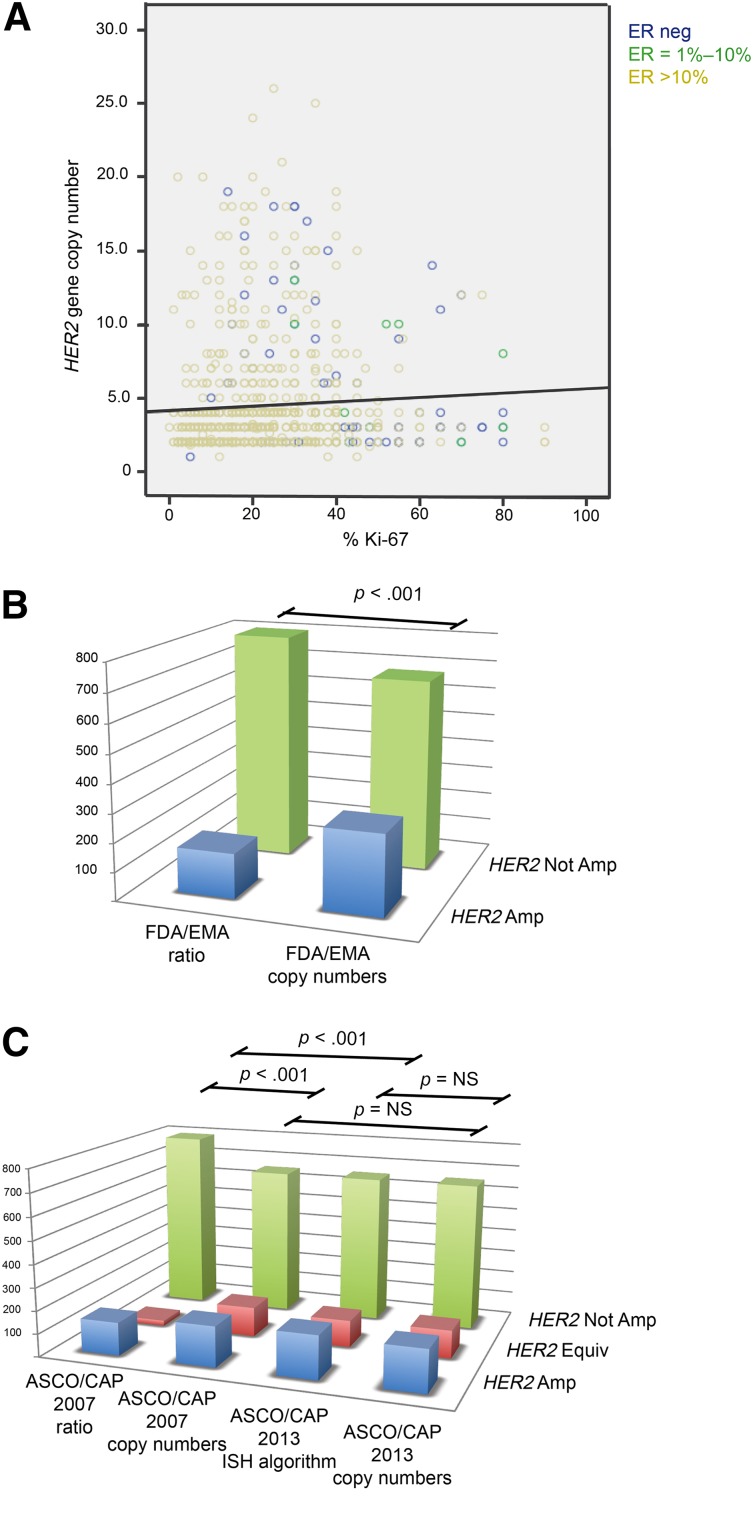
Figure 1.
Distribution of immunophenotypical variables throughout the cohort and prevalence of amplification according to distinct guidelines. (A): Relationship of ER, Ki-67, and HER2 gene copy number values in immunohistochemistry-evaluated HER2-equivocal carcinomas. These carcinomas are largely ER positive (green circles between 1%–10%, yellow circles when >10% of positive cells) with high proliferative activity; however, presence of amplification is associated with ER-negative carcinomas (blue circles exhibiting high HER2 copy numbers). A great proportion of ER-positive cases with low proliferation indexes also harbor a low HER2 gene copy number. (B, C): Statistically significant differences in the distribution of HER2 amplified, not amplified, and equivocal cases (the latter for ASCO/CAP only) are observed with the different scoring methods of the FDA/EMA and ASCO/CAP recommendations, as shown by p values. Notably, no statistically significant differences were observed between the scoring of dual- and single-signal assays according to ASCO/CAP 2013 and between HER2 copy number by ASCO/CAP 2007 and both ISH algorithm and HER2 copy number by ASCO/CAP 2013.
Abbreviations: Amp, amplified; ASCO/CAP, American Society of Clinical Oncology/College of American Pathologists; EMA, European Medicines Agency; Equiv, equivocal; ER, estrogen receptor; FDA, U.S. Food and Drug Administration; ISH, in situ hybridization; neg, negative; Not Amp, not amplified; NS: not statistically significant.