Table 2. Cycloaddition of Benzylazide with Various Benzynesa.
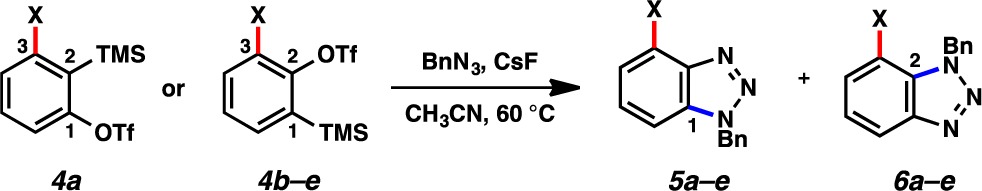
| entry | 4a–e | computed ΔΔG⧧ (ratio 5:6) | experimental yield (ratio 5:6)b |
|---|---|---|---|
| 1a | 4a, X = OMe | 3.4 kcal/mol (>292:1) | 94% (5a formed exclusively) |
| 2 | 4b, X = F | 2.5 kcal/mol (>71:1) | 68% (5b formed exclusively) |
| 3 | 4c, X = Cl | 1.4 kcal/mol (>10:1) | 53% (>16:1) |
| 4 | 4d, X = Br | 1.2 kcal/mol (>8:1) | 45% (12:1) |
| 5 | 4e, X = I | 0.9 kcal/mol (>5:1) | 43% (6:1) |
Conditions: see Supporting Information. Computed ratios obtained from Boltzmann factors using B3LYP/6-31G(d) free energies including CPCM solvation by MeCN; methylazide was used as a model for benzylazide to simplify computational studies.
Experimental yields and ratios are the average of three experiments and were determined by 1H NMR analysis using hexamethylbenzene as an external standard.
