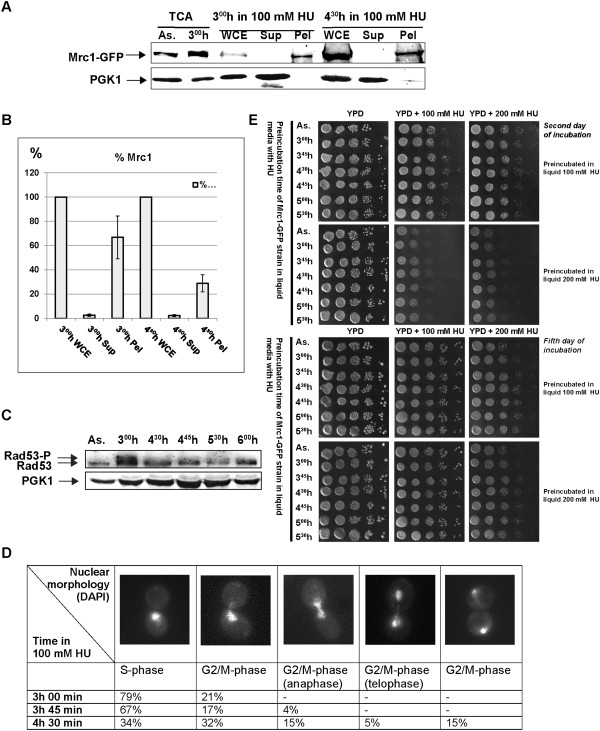
Figure 5.
Mrc1-GFP cells by-pass the S-phase checkpoint. (A) Bulk chromatin fractioning assay of Mrc1-GFP strain in the presence of 100 mM HU. Samples from indicated time points were fractionated into whole cell extract (WCE), crude soluble (Sup) and chromatin (Pel) fractions. Yeast total protein extracts (TCA) from asynchronous (As.) and treated for three hours (300 h) with 100 mM HU MRC1-GFP cells were run on the same 6–15% SDS-PAGE. As a control, the cytoplasmic PGK1 protein was monitored on the same Western blotting membrane. (B) The average amount of Mrc1-GFP protein from different Bulk chromatin fractioning assays, measured by the Gel analysis tool of ImageJ software. The measured amount of Mrc1-GFP from the WCE of each time point is assumed as 100%, the protein from Sup and Pel is calculated as a percentage of respective WCE. Standard deviation of means is indicated as error bars. (C) Total protein extracts from indicated time points from Mrc1-GFP cells in the presence of 100 mM HU. Samples were run on a 6–15% SDS-PAGE and after western blotting, immunodetections of Rad53 and PGK1 (loading control) were carried out. Rad53-P and Rad53 indicate the phosphorylated and unphosphorylated forms of Rad53 protein. (D)Mrc1-GFP strain nuclear morphology analysis of treated with 100 mM HU yeast cells. Samples from indicated time points were DAPI stained and monitored under fluorescent microscope. The number of cells with indicated nuclear morphology is given as a percentage of the sum of all counted cells from each sample. (E) Exponentially growing S. cerevisiae cells from MRC1-GFP strain were arrested with 100 mM HU or 200 mM HU for 3 h in liquid YPD medium and then plated on YPD and YPD, containing 100 or 200 mM HU respectively at indicated time points. The duration of incubations is indicated.