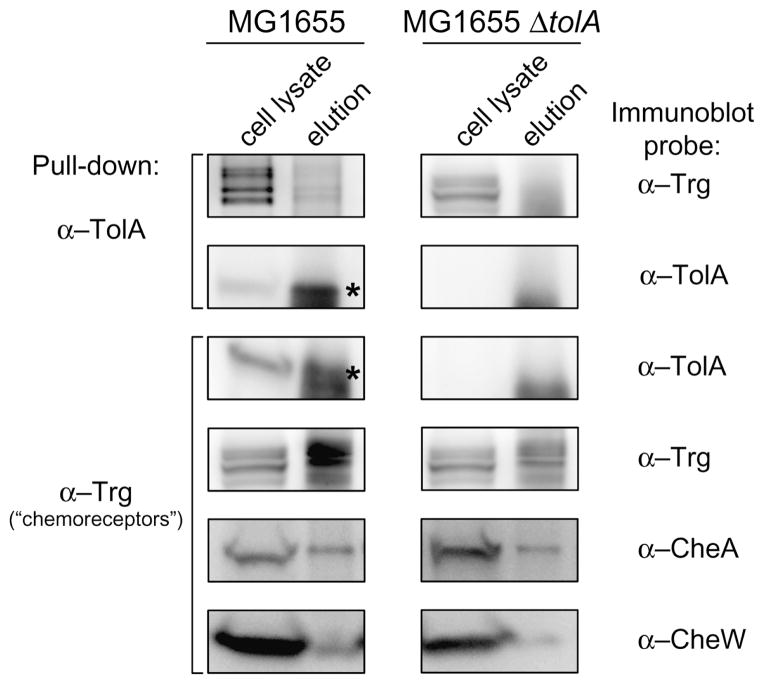
Fig. 5.
The inner membrane protein TolA interacts with chemoreceptors. In vivo co-immunoprecipitation (co-IP) of TolA in wild-type E. coli MG1655 and in the isogenic mutant E. coli MG1655 ΔtolA. Western blots of whole-cell lysate and elution fraction of samples immunoprecipitated with anti-TolA (α-TolA) (indicated on the left) were probed with anti-Trg (α-Trg) and with α-TolA (indicated on the right). The reverse co-IP was performed with whole-cell lysate samples immunoprecipitated with α-Trg (indicated on the left) and probed with α-TolA. As a control, Western blots of whole-cell lysate and elution fraction of samples immunoprecipitated with α-Trg were separately probed with anti-CheA (α-CheA), anti-CheW (α-CheW), and α-Trg. The α-Trg is an antiserum raised to highly purified E. coli Trg; however, it also recognizes the other E. coli chemoreceptors (Tar, Tsr, and Tap) (Morgan et al., 1993). Because cross-reactivity of α-TolA with unknown components of the co-IP eluate generated another signal close to the band of interest (TolA), asterisks were added to facilitate visualization of TolA.