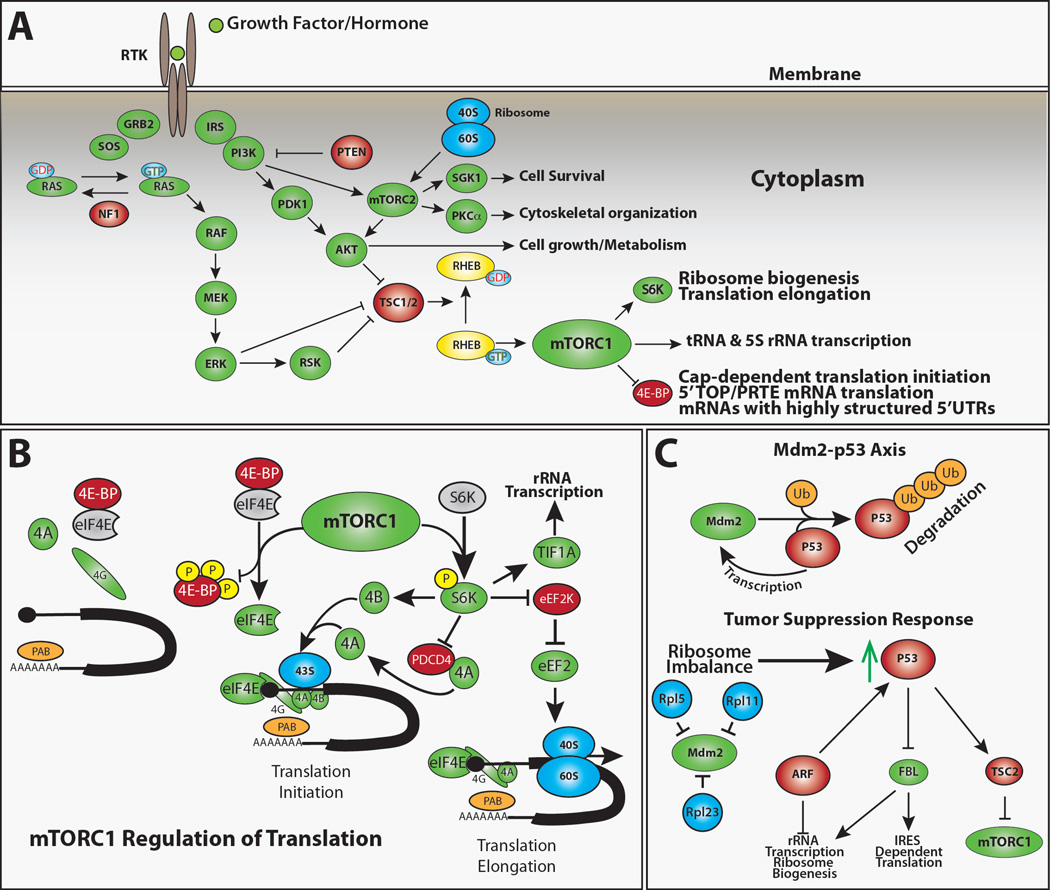
Figure 2. Signaling pathways that regulate protein synthesis.
(A) Growth factors, hormones, nutrients, and stress all influence protein synthesis through complex signaling pathways (Laplante and Sabatini, 2012; Topisirovic and Sonenberg, 2011). mTORC1 preferentially promotes the translation of a subset of mRNAs that contain complex 5’UTRs and clusters of 5’ terminal oligopyrimidines (TOP) or similar pyrimidine-rich sequences (Hsieh et al., 2012; Jefferies et al., 1994; Thoreen et al., 2012). These TOP genes primarily encode proteins that promote translation, including ribosomal proteins, initiation factors, and elongation factors (Thoreen et al., 2012). While many proteins have been proposed to regulate the translation of TOP mRNAs (Damgaard and Lykke-Andersen, 2011; Tcherkezian et al., 2014) and the translation of specific TOP containing mRNAs is regulated in a cell-specific manner (Avni et al., 1997; Ivanov et al., 2011), the roles of these elements in stem cell maintenance and tissue homeostasis remain unclear.
(B) The regulation of translation initiation represents the major mechanism by which cells regulate protein synthesis. The eIF4F complex, which includes eIF4E, eIF4G and eIF4A, promotes the translational initiation of virtually all cellular mRNAs (Sonenberg and Hinnebusch, 2009) and mRNAs with highly structured 5’UTRs are particularly sensitive to eIF4E activity (Feoktistova et al., 2013; Koromilas et al., 1992). Activated mTORC1 phosphorylates 4E-BPs, preventing their inhibition of eIF4E. In the absence of 4E-BPs, eIF4E binds to the m7G mRNA cap and recruits eIF4G, which in turn acts as a scaffold for the RNA helicase eIF4A. The formation of this eIF4F complex on the 5’-end of mRNA promotes the initiation of cap-dependent translation. Activated mTORC1 also phosphorylates and activates S6K, which phosphorylates eIF4B, eEF2K and TIF1A. Phosphorylated eIF4B enhances the helicase activity of eIF4A to unwind secondary structures in mRNA, which promotes translational initiation. Phosphorylation of eEF2K by S6K interferes with its ability to block the activity of the translational elongation factor eEF2. Activation of TIF1A promotes ribosomal RNA transcription.
(C) The E3 ubiquitin ligase Mdm2 promotes the degradation of the tumor suppressor p53. p53 acts in a feedback loop to promote the transcription of the Mdm2 gene. Thus under normal conditions, p53 levels remain low. Imbalances in ribosome biogenesis induce a tumor suppressor response in which free ribosomal proteins, particularly Rpl5 and Rpl11, bind and inhibit Mdm2 (Bhat et al., 2004; Dai and Lu, 2004; Dai et al., 2006; Zhang et al., 2003). This results in the accumulation of p53, which promotes the activity of TSC2, thereby repressing the activity of mTORC1. p53 and other tumor suppressors, such as ARF, inhibit rRNA transcription and ribosome biogenesis (Lessard et al., 2010; Sloan et al., 2013; Sugimoto et al., 2003).