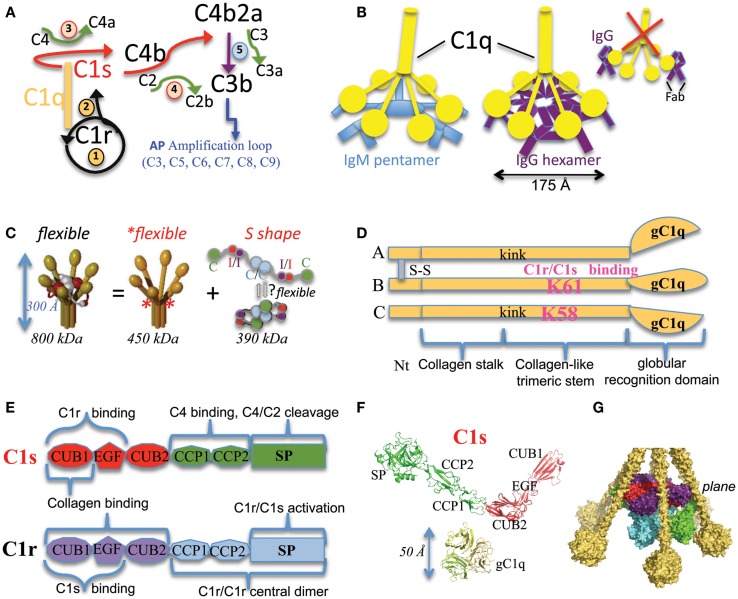
Figure 1.
Functional and structural elements of the CP activation. (A) The main steps of the complement activation cascade through the CP. The multimeric C1q molecule is associated to the C1s–C1r–C1r–C1s tetramer. When C1q binds to an activating target surface, a conformational change triggers the auto-activation of the associated C1r protease (converting the pro-enzyme into an activated form, black circular arrow), which then activates C1s (black arrow). C1s activates C4 and C4b-bound C2 (red arrows), leading to the assembly of the classical C3 convertase C4b2a. Green arrows are used for the activation cleavage of C2, C3, and C4, with the release of a small fragment. Details of the consecutive AP amplification loop are not given for sake of clarity. It involves C3–C9 components and mediates rapid opsonization, signaling events, as well as eventually formation of the lytic pore. The initial steps are numbered from 1 to 5. The first two steps occur inside C1 and depend on C1q conformational change and the consequent C1r activation. Steps 3 and 4 depend on C1s proper positioning and catalytic activity. (B) Current hypothetical schemes on similar interaction modes between C1q and IgM or IgG hexamers, the best CP activators. The new scheme proposed for IgG is in contrast with the traditional old scheme (right) depicting one C1q molecule interacting with two distant IgG molecules, each antigen-bound through its two Fab arms. (C) The “C1 paradox” and initial low resolution C1 models. C1 is a 30 nm high multimer resulting from the association of the flexible recognition protein C1q with the flexible C1s–C1r–C1r–C1s tetramer, which appears more elongated (S extended shape) in solution than in the complex (thus the initial “paradox”). C1q (yellow) has a hexameric shape, built from 18 chains. Interaction (I) and catalytic (C) domains of C1r and C1s are labeled and colored on the right side. The asterisks show the position of flexible hinges in C1q. The low resolution model on the left and the proposed tetramer conformational equilibrium on the right are derived from (3). (D) Modular structure of each C1q chain type. A, B and C chains associate as a hexamer of ABC heterotrimers. Kink indicates the position of disruptions in the triplets occurring only within collagen-like sequences of the A and C chains and probably inducing flexible hinges. The disulfide bridging between chains A and B is illustrated. The C chain has no covalent link with A and B chains, but covalently associates pairs of ABC trimers through a C–C disulfide bridge. The two lysines crucial for C1 assembly are shown in pink. (E) Modular structure and associated functions of C1r and C1s. The catalytic domain includes the C-terminal serine protease (SP) domain as well as the preceding Complement Control Protein (CCP or sushi) modules. The interaction domains of C1s and C1r involve their N-terminal CUB–EGF–CUB modules. The corresponding functional implications are mentioned. The same color coding is used in (F,G) and in the right panel of (C). “CUB” means initially found in Complement C1r and C1s, Uegf and BMP-1. (F) C1 is a large complex made of small building blocks of (mainly) known structures. The displayed C1s is a composite structure obtained after superposing the PDB structures 1ELV (4) and 4LMFA (5) onto 4LOT (5) (see details in Table S1 in Supplementary Material). The color code used is the same as in (C,E). The chains ABC from the C1q globular domain [2WNV (6)] are shown on the same scale. (G) Example side view of a partial composite C1 model, refined using the results of differential accessibility in C1q and C1 using chemical lysine labeling followed by mass spectrometry (7). The C1r and C1s proteases interact with C1q through their interaction domains aligned on the same plane (which corresponds to the position of LysB61 and LysC58 in C1q). This part of the model is mainly confirmed by recent complementary experimental studies (8). The position of the catalytic domain of C1s is more uncertain and probably variable.