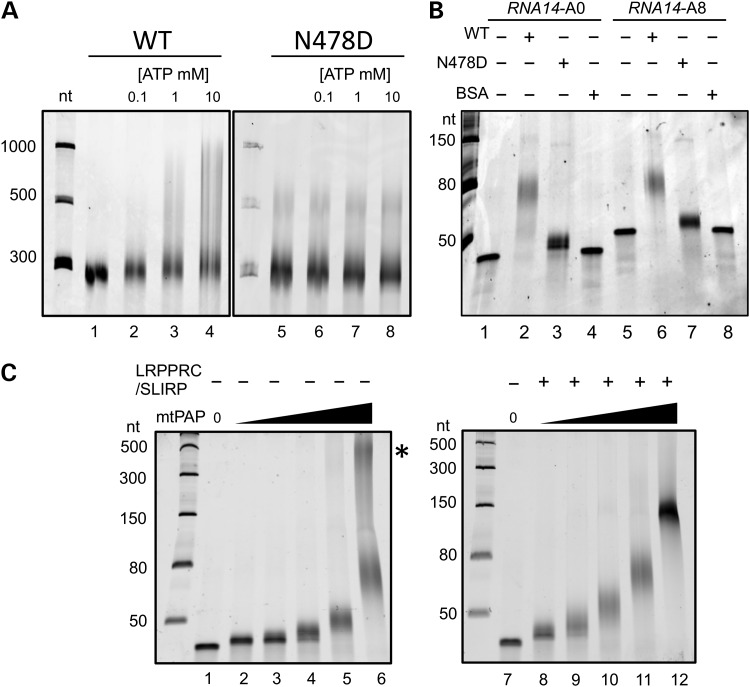
Figure 4.
In vitro polyadenylation activity of mtPAP. (A) Polyadenylation activity of recombinant wild-type (WT; lanes 2–4) and mutant (p.N478D; lanes 6–8) mitochondrial poly(A) polymerase (0.55 µm) was determined with increasing ATP concentrations. The RNA substrate was an unadenylated 277-nt 3′ fragment of MTND3 (0.25 µm). The right hand panel contains an IVT RNA artefact (500 nt) present in the absence of mtPAP (lane 5). Reactions were quenched with 90% formamide/1× TBE, separated through a 6% polyacrylamide/8.3 m urea gel, then stained with SYBR gold and visualized by scanning with a Typhoon FLA 9500 instrument. (B) Short RNAs (0.25 µm) corresponding to the final 40 nucleotides of RNA14 with (A8, lanes 5–8) or without (A0, lanes 1–4) an oligo(A8) addition were used as templates for polyadenylation by recombinant wild-type (WT; lanes 2 and 6) or mutant (p.N478D; lanes 3 and 7) mtPAP (0.55 µm). An equal amount of BSA was added in a parallel experiment as a control (lanes 4 and 8). Products were separated through 15% polyacrylamide/8.3 m urea gel and visualized as in (A). (C) Increasing amounts of wild-type mtPAP (34 nm to 0.55 µm) were added to the short RNA14A0 (0.25 µm) template in the presence (lanes 8–12) or absence (lanes 2–6) of LRPRRC/SLIRP complex. A higher molecular species (*) of varying intensity was observed with wild-type mtPAP. Products were separated and visualized as in (B).