Table 3.
Synthesis of highly substituted 1H-imidazo[1,2-b]pyrazolesa.
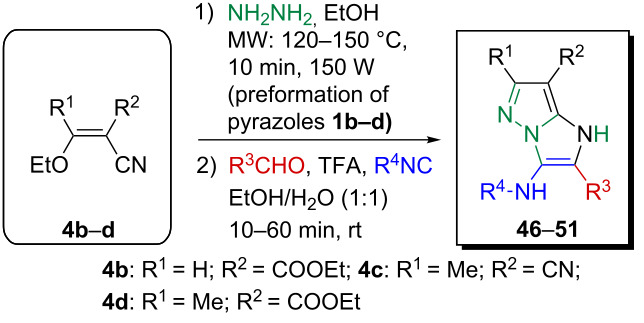 | ||||||
| Entry | R1 | R2 | R3CHO | R4NC | Product | Yieldb (%) |
| 1 | H | COOEt | 2a | 3a | 46 | 54 |
| 2 | H | COOEt | 2b | 3b | 47 | 56 |
| 3 | Me | CN | 2a | 3a | 48 | 79 |
| 4 | Me | CN | 2c | 3c | 49 | 57 |
| 5 | Me | COOEt | 2a | 3a | 50 | 74 |
| 6 | Me | COOEt | 2i | 3b | 51 | 59 |
aReaction conditions: 4b–d (0.50 mmol), 5 (0.55 mmol), ethanol (0.5 mL), MW (10 min; 4b: 150 °C, 4c,d: 120 °C; 150 W), then water (0.5 mL), 2a–c,i (0.55 mmol), TFA (0.10 mmol), 3a–c (0.55 mmol), room temperature, 10–60 min. bIsolated yield after simple filtration.
