Abstract
The introduction of reduced intensity/toxicity conditioning regimens has allowed allogeneic hematopoietic cell transplantation to be performed in patients who were previously considered too old or otherwise unfit. Although it led to a reduction in non-relapse mortality, disease control remains a major challenge. We studied the outcome of 165 patients with acute myeloid leukemia (n=124) or myelodysplastic syndrome (n=41) transplanted after conditioning with fludarabine (30 mg/m2/day for 5 days), intravenous busulfan (either 260 mg/m2: reduced intensity conditioning, or 390–520 mg/m2: reduced toxicity conditioning), and rabbit anti-thymoglobulin (2.5 mg/kg/day for 2 days). The median age of the patients at transplantation was 56.8 years. The 2-year relapse incidence was 29% (23% versus 39% for patients transplanted in first complete remission and those transplanted beyond first complete remission, respectively; P=0.008). The 2-year progression-free survival rate was 57% (95% CI: 49.9–65). It was higher in the groups with favorable or intermediate cytogenetics than in the group with unfavorable cytogenetics (72.7%, 60.5%, and 45.7%, respectively; P=0.03). The cumulative incidence of grades 2–4 and 3–4 acute graft-versus-host disease at day 100 was 19.3% and 7.9%, respectively. The cumulative incidence of chronic graft-versus-host disease at 1 year was 21.6% (severe forms: 7.8%). Non-relapse mortality at 1 year reached 11%. The 2-year overall survival rate was 61.8% (95% CI: 54.8–69.7). Unfavorable karyotype and disease status beyond first complete remission were associated with a poorer survival. This well-tolerated conditioning platform can lead to long-term disease control and offers possibilities of modulation according to disease stage or further development.
Introduction
Allogeneic hematopoietic stem cell transplantation is currently the most effective treatment in eligible patients with acute myeloid leukemia (AML) or high-risk myelodysplastic syndromes (MDS).1 This approach was initially reserved for young, fit patients, given the high toxicity related to myeloablative conditioning regimens.2 Older patients were usually not considered for standard myeloablative allogeneic hematopoietic stem cell transplantation because of additional toxicities due to age and associated comorbidities. However, the incidence of AML and MDS tends to increase with age.3 Moreover, even in younger patients, standard myeloablative conditioning regimens cannot be performed safely in cases of comorbidities that would lead to a higher risk of non-relapse mortality. Therefore, most AML or MDS patients with potential indications could not benefit from allogeneic hematopoietic stem cell transplantation. So-called non-myeloablative or reduced-intensity conditioning regimens have been developed in order to reduce the non-relapse mortality.4–7 This approach focuses on the allogeneic graft-versus-leukemia effect8 and was thought to allow disease control, despite the reduction of the cytotoxicity of the antileukemic conditioning, while decreasing transplant-related morbidity and mortality. Early reports were encouraging and non-myeloablative and reduced-intensity conditioning regimens are now widely used. However, several retrospective studies have raised concerns about disease control when reducing the conditioning intensity.9–14 Indeed, it is probable that the choice of a conditioning regimen should be based not only on the patients’ characteristics (age and comorbidities) but also on disease type and stage6,15–17 and prognostic factors (mainly cytogenetic and molecular markers in myeloid malignancies15,18). Myeloablative reduced-toxicity regimens that combine truly myeloablative doses of alkylating agents or intermediate doses of total body irradiation and highly immunosuppressive agents have been developed to achieve better disease control with an acceptable non-relapse mortality.19–21 However, the ideal conditioning regimen has not yet been found. The association of busulfan and fludarabine has been widely used.19,22 We initially reported that this association, together with a low dose of rabbit anti-thymoglobulin, held some promise.22 However, the low dose of rabbit anti-thymoglobulin was associated with relatively high incidences of acute and chronic graft-versus-host disease (GVHD), which affected survival. We therefore increased the dose of rabbit anti-thymoglobulin to reduce the incidence of GVHD without increasing the relapse rate.23 The refinement of rabbit anti-thymoglobulin and intravenous (i.v.) busulfan doses in combination with fludarabine allowed us to develop a safe and flexible reduced-intensity/toxicity conditioning platform. In this paper, we describe the outcome of 165 high-risk AML and MDS patients treated with this conditioning.
Methods
Patients’ and disease characteristics
Patients from two associated French transplantation centers transplanted between January 2005 and December 2011 were eligible if they: (i) were adult patients (≥18 years); (ii) had de novo or secondary AML or MDS; (iii) received a reduced-intensity or reduced-toxicity conditioning regimen including fludarabine, i.v. busulfan and rabbit anti-thymoglobulin 5 mg/kg; (iv) had an HLA-identical matched related, or matched or mismatched unrelated donor; and (v) received peripheral blood or bone marrow stem cells as the graft.
Clinical and biological data were prospectively collected from a shared patients’ database and were crosschecked with information from individual institutional clinical files. Morbidity and mortality risks related to transplantation were assessed by the comorbidity index described by Sorror et al.24 Patients were considered to be at high risk when their score was ≥3.25
Disease status at transplantation was defined according to previously described international criteria.26,27 Karyotype was defined as favorable, intermediate, and unfavorable as described by Döhner et al.27
FMS-like tyrosine kinase 3/internal tandem duplication (FLT3-ITD), nucleophosmin 1 (NPM1) and CCAAT/enhancer binding protein alpha (CEBPα) mutation status was available for 47 cases among 81 AML patients with an intermediate karyotype.
Ethical committee approval
All patients gave informed consent to clinical investigations; according to standard practice in France both institutional review boards approved the retrospective design of the study.
Transplant characteristics
All patients received a conditioning regimen including fludarabine, i.v. busulfan and rabbit anti-thymoglobulin 5 mg/kg. A conditioning regimen using 2 days of busulfan (total dose 260 mg/m2) was defined as reduced-intensity conditioning, whereas regimens involving 3 or 4 days of busulfan (390 mg/m2 and 520 mg/m2, respectively) were defined as reduced-toxicity conditioning.28 The total dose of busulfan was determined by the attending physician who took into consideration the patient’s age and comorbidities. Supportive care was performed as previously described.22 Acute and chronic GVHD were graded according to the National Institutes of Health classification.29,30
For details, see the Online Supplementary Methods.
Statistical analyses
The outcomes of interest were cumulative incidence of relapse, non-relapse mortality, overall survival, and progression-free survival, and were determined from the date of transplantation. When AML relapsed or progressed, relapse/progression was considered as a cause of death regardless of other events. All deaths without relapse/progression were considered non-relapse mortality. Death from any cause was a relevant event for overall survival. Events for progression-free survival were relapse or death from any cause. Overall survival and progression-free survival rates were estimated using the Kaplan–Meier method,31 and the log-rank test was applied to compare survival probabilities between subgroups of patients. Death without evidence of progression was treated as a competing risk in the analyses of cumulative incidence of relapse, whereas death following relapse or progression was treated as a competing risk when analyzing non-relapse mortality.32 The Prentice estimation and Gray test were used to analyze cumulative incidence of relapse and non-relapse mortality. Hazard ratios (HR) and their 95% confidence intervals (95% CI) were estimated with Cox proportional hazard (overall survival, progression-free survival) and Fine and Gray (cumulative incidence of relapse and non-relapse mortality) models. All statistical analyses were performed using R.2.14.1 statistical software and SPSS (SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
The study population comprised 165 patients with AML (n=124) or MDS (n=41) who met the inclusion criteria. The median age of the patients at transplantation was 56.8 years (range, 18–71). Disease type, disease status at time of transplantation, karyotype, donor type, stem cell source and conditioning intensity (i.e., total dose of busulfan) are presented in Table 1.
Table 1.
Patients’ and transplant characteristics.
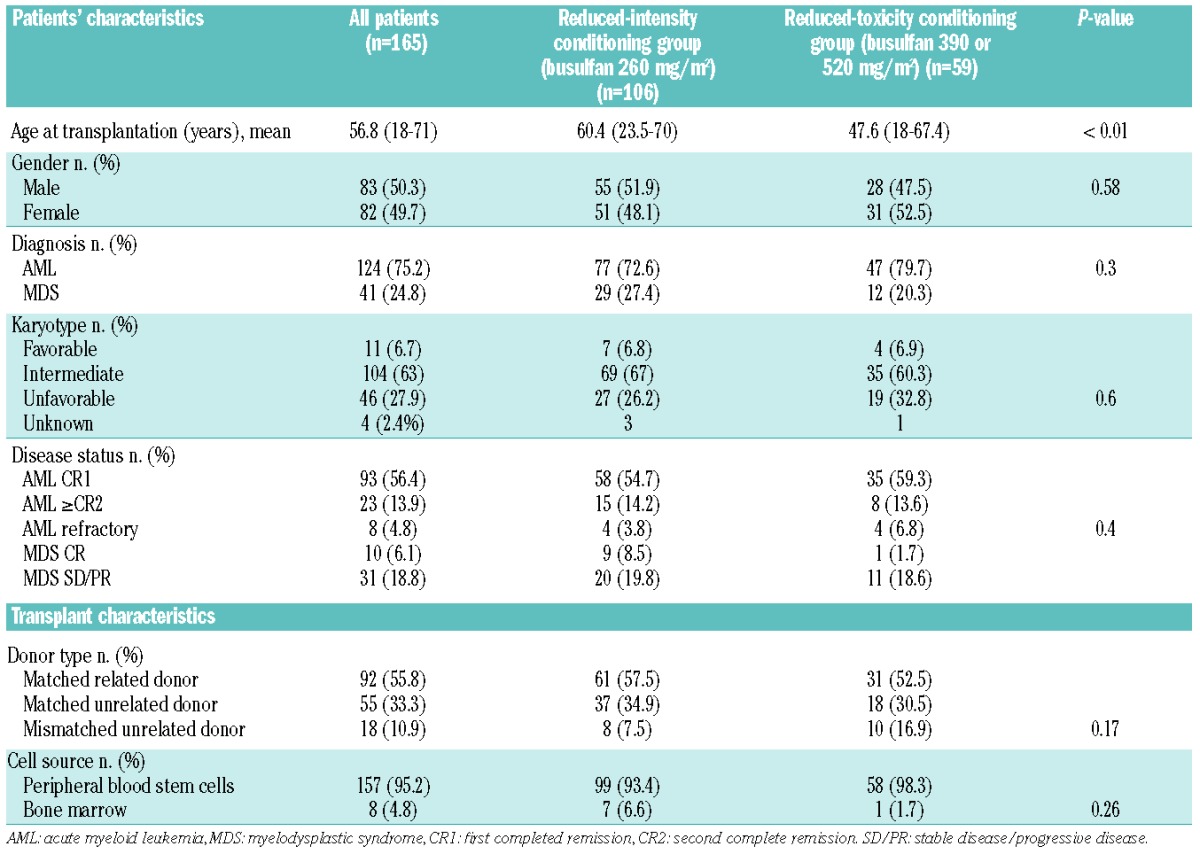
Comorbid conditions assessed by the comorbidity index score described by Sorror et al. were determined in 58% of the patients: 71% of patients had a score ≥2, and 47.1% had a score ≥3. There was no significant difference in scores between patients under or over 55 years of age. Additionally, we did not observe a significant difference in the comorbidity score between the two conditioning groups (score ≥2 in 72% of cases in the busulfan 260 mg/m2 group versus 68% in the 390 mg/m2 or 520 mg/m2 group). Patients over 55 years old at the time of transplantation (n=89; 53.9%) received reduced-intensity conditioning (busulfan = 260 mg/m2) in most cases (75/89; 84.3%), although 14 received reduced-toxicity conditioning [busulfan 390 mg/m2 in 11 (12.4%), and bulsulfan 520 mg/m2 in 3 (3.4%) cases]. Patients under 55 years of age (n=76) received 260 mg/m2, 390 mg/m2, and 520 mg/m2 of busulfan intravenously in 31 (40.8%), 24 (31.6%), and 21 (27.6%) cases, respectively.
Post-transplant outcomes
Engraftment
Full engraftment occurred in all patients except for one. This patient with MDS had progressive secondary graft failure. He is still alive 25 months after transplantation and is receiving adapted supportive care.
Relapse and progression-free survival
Overall, 56 patients relapsed, within a median time after transplantation of 164.5 days (range, 17–1520). The 1- and 2-year cumulative incidences of relapse were 24% and 29%, respectively. Three patients received a second allogeneic hematopoietic stem cell transplant after the relapse. Patients beyond first complete remission had a higher 2-year cumulative incidence of relapse (23% versus 39% for first complete remission and non-first complete remission patients, respectively; P=0.008). There was no difference in relapse rates considering the total dose of busulfan, the donor type, the cytogenetic status or age under or over 55 years (Table 2). After adjusrment for other variables, the prognostic value of disease status remained the only significant value (HR 2.32, 95% CI: 1.23–4.39 for patients not in first complete remission; P=0.009)
Table 2.
Univariate analysis.
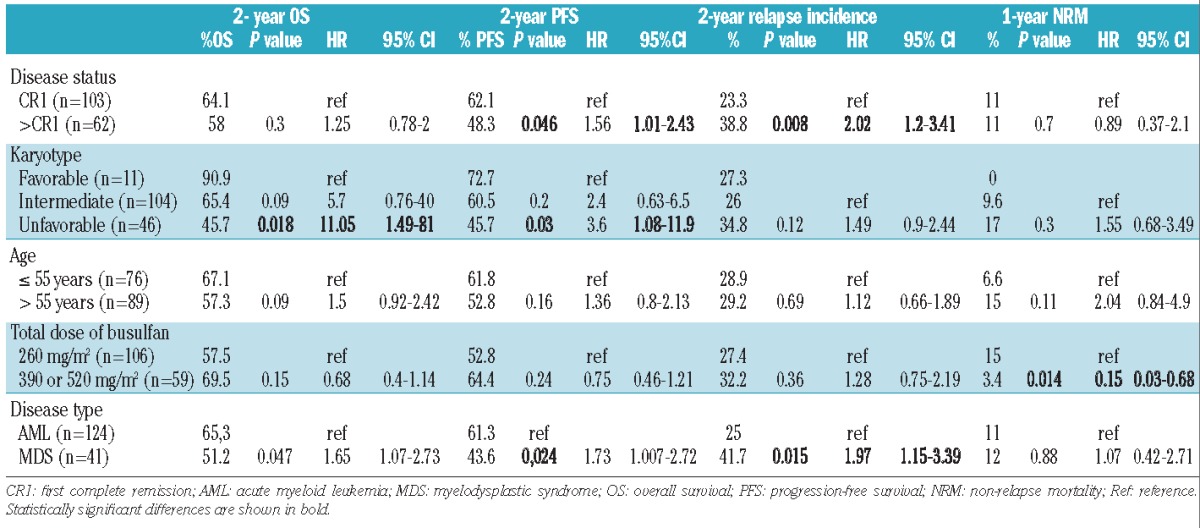
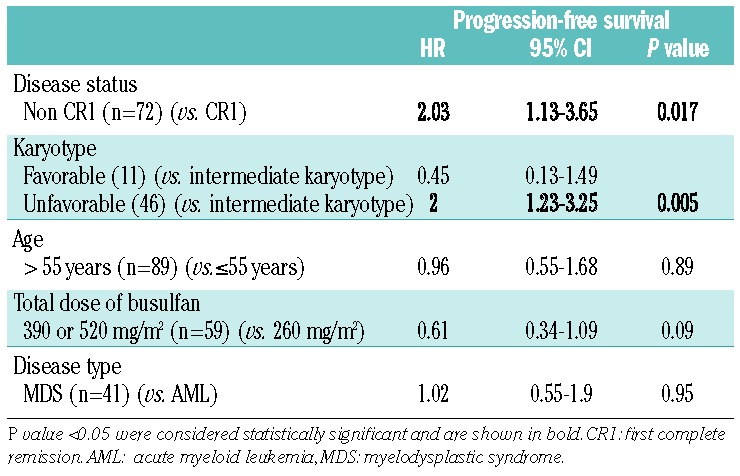
The 2-year progression-free survival rate was 57% (95% CI: 49.9–65) (Figure 1A). The rate was higher in the groups with favorable or intermediate cytogenetics than in the group with unfavorable cytogenetics (72.7%, 60.5%, and 45.7%, respectively; P=0.03) (Table 2). Patients beyond first complete remission had a lower progression-free survival rate (48.3% versus 62.1%; P=0.04). Age at transplantation, disease type, and conditioning had no impact on progression-free survival. Disease status beyond first complete remission and adverse karyotype remained significantly associated with lower progression-free survival rates after adjustment for other variables (Table 3).
Figure 1.
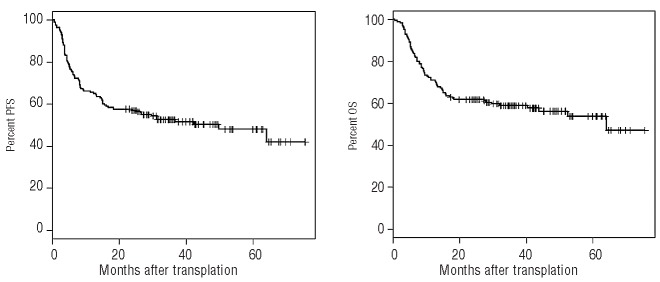
(A) Progression-free survival (PFS) for the whole cohort, n=165. (B) Overall survival (OS) for the whole cohort, n=165.
Table 3.
Multivariate analysis for progression-free survival.
Non-relapse mortality
The non-relapse mortality rates at 3, 12, and 24 months after transplantation were 2%, 11%, and 14%, respectively. The patients who received reduced-toxicity conditioning (i.v. busulfan 390 mg/m2 or 520 mg/m2) had a lower non-relapse mortality (3.4% at 24 months versus 19.9% for the remaining patients; P=0.003). However, these patients were younger (median age: 46.5 versus 57.6 years; P=0.001).
The main cause of non-relapse mortality was acute or chronic GVHD (15 cases). Fatal infection without GVHD occurred in four cases. One patient died from secondary neoplasm, one from relapse of primary breast cancer, two from heart failure, one from an accidental fall, and one from sinusoid obstruction syndrome.
We found no impact of donor type, disease status, age under or over 55 years or comorbidity index score on non-relapse mortality (Table 2).
Acute graft-versus-host disease
Patients developed acute GVHD at a median time of 35 days after hematopoietic stem cell transplantation (range, 8–153 days). The cumulative incidence of grades 2–4 acute GVHD was 19.3% at day 100. There was no significant difference between patients younger and older than 55 years (13.1% versus 24.7% at day 100, respectively; P=0.1). The cumulative incidence of grades 3–4 acute GVHD was 7.9% at day 100. Patients over 55 years had a higher cumulative incidence of grades 3–4 acute GVHD than the younger patients (12.4 versus 2.6% at day 100, respectively; P=0.05). Neither donor type nor total dose of busulfan had any significant impact on acute GVHD.
Chronic graft-versus-host disease
The cumulative incidences of chronic GVHD (all grades) were 15.1% and 21.8% at 6 and 12 months, respectively. The cumulative incidences of severe chronic GVHD (National Institutes of Health classification) were 5.4% and 7.8% at 6 and 12 months, respectively. There was no difference in GVHD incidences between patients divided according to age at transplantation (under or over 55 years), donor type, comorbidity index or busulfan dose.
Among patients suffering from chronic GVHD who survived more than 1 year after transplantation (n=35), 19 were able to discontinue steroid therapy for chronic GVHD before 1 year after the transplant (54.3%).
Overall survival
At the time of the last follow-up, 93 (56.4%) patients were alive. The median follow-up duration for living patients was 38.9 months (range, 19.8–75.7 months). The main cause of death was disease relapse/progression (n=47; 65.3%).
The 2- and 5-year overall survival estimates were 61.8% (95% CI: 54.8–69.7) and 53.8% (95% CI: 45.5–63.6), respectively (Figure 1B). Age beyond 55 years was not statistically associated with a poorer overall survival.
Unfavorable karyotype and disease status beyond first complete remission were significantly associated with a poorer overall survival (Table 2). In multivariate analysis, the cytogenetic status was the only factor that remained statistically significant (HR 2.2; 95% CI: 1.33–3.65 for unfavorable karyotype; P=0.002).
Discussion
The association of fludarabine, busulfan, and rabbit anti-thymoglobulin as a platform for a conditioning regimen has been used for more than a decade in our institutions, with encouraging results after continuous optimization.7,22,33 We recently showed that within this association, increasing the dose of rabbit anti-thymoglobulin from 2.5 mg/kg to 5 mg/kg reduced the incidence of acute and chronic GVHD without increasing the relapse rate.23 Here, we aimed to evaluate the results of this combination in terms of disease control in a larger group of patients with myeloid malignancies.
Our patients were characterized by a relatively high risk of relapse: 37.6% were beyond first complete remission and 27.9% had an unfavorable karyotype. However, the cumulative incidences of relapse and progression-free survival were promising, 29.1% and 57%, respectively, at 2 years. These results compare favorably with those of previous studies based on reduced toxicity conditioning (2-year progression-free survival: 44%),34 or even myeloablative conditioning regimens (progression-free survival between 31% and 54%),9–11 in patients with conditions rather similar to those in our study. Our results are also noteworthy if compared with those of studies based on non-myeloablative conditioning (progression-free survival ranging from 32% to 44% in two large recent studies).6,18 In brief, our conditioning platform allows for substantial disease control, as compared with that achieved in previous studies.
Despite the patients’ older ages and high rate of comorbidities, non-relapse mortality in this cohort was low: 2.4% at day 100 and 14% at 2 years, likely due to a low incidence of severe acute and chronic GVHD. These results are similar to the non-relapse mortality reported in patients undergoing true non-myeloablative conditioning regimens (1-year non-relapse mortality under 16%),16,18,35 which are known to be associated with lower non-relapse mortality than that in myeloablative conditioning regimens. If compared with studies based on reduced-toxicity conditioning, our results were even more satisfactory (1-year non-relapse mortality: 19% in a recent study by Alatrash et al.).34
Taken together, the relatively low non-relapse mortality and relapse incidence led to a 61.8% overall survival at 2 years, which compares favorably with results from the above-cited studies including reduced-intensity conditioning (overall survival between 26% and 60%)5,10,12,15,36–40 and myeloablative conditioning (overall survival between 35% and 56%).11,34,41
Our patients received either reduced-intensity conditioning or reduced-toxicity conditioning. A comparison between the two groups did not reveal significant differences regarding disease type, disease status, donor type, or stem cell source although there was a difference in age at transplantation (Table 1). Surprisingly, we found a lower non-relapse mortality in the reduced-toxicity conditioning group (busulfan dose: 390 mg/m2 or 520 mg/m2). However, due to the retrospective nature of this study, it cannot be concluded that more intensive conditioning leads to lower non-relapse mortality rates. The choice of the busulfan dose was, in fact, based on physicians’ assessment, and many patients were included in trials at the time in which physicians were advised to reserve higher doses of busulfan for patients under 55 years of age or who had no comorbidities. The indications for more intensive conditioning were extended to older patients and those with comorbidities when it became evident that the regimens were well tolerated. The difference in age at transplantation between the two groups could also explain the difference in non-relapse mortality rates: patients who received a lower busulfan dose were older (median age 60.4 years) than those who received more intensive conditioning (median age 47.6 years; P<0.001). Only a prospective, randomized study would be able to show whether or not more intensive conditioning is associated with a higher non-relapse mortality. We can, however, conclude that higher doses of busulfan were not associated with greater toxicity in selected patients.
We did not detect any significant differences in disease control between patients given reduced-intensity or reduced-toxicity conditioning. This could be explained by the retrospective nature of our study and by the fact that reduced-toxicity conditioning was often chosen by clinicians in cases of diseases with higher risk of relapse, based on previous studies showing a higher relapse rate when using non-myeloablative conditioning regimens.4,11,12 However, the impact of greater myeloablation on relapse rates deserves further prospective evaluation.
Patients received an intermediate dose of rabbit anti-thymoglobulin (5 mg/kg). This in vivo T-cell depletion has been controversial. Its use has been shown to decrease GVHD incidence in several studies23,37,42–44 without increasing overall survival or progression-free survival, perhaps because of an impaired graft-versus-leukemia effect. Given that our patients were at high risk of GVHD and non-relapse mortality because of their ages and comorbidities, the use of rabbit anti-thymoglobulin was of particular interest. Our results support previous data suggesting that a dose of 5 mg/kg does not dramatically increase relapse rates. Moreover, we determined that most patients (54%) who suffered from chronic GVHD and who were alive more than 1 year after transplantation were able to discontinue steroid therapy within 1 year, which reflects the favorable outcome of their chronic GVHD. Contrary to previously published observations, we did not see a high proportion of severe, fatal infections;45 only four patients died from infection without GVHD. A dose of 5 mg/kg of rabbit anti-thymoglobulin may, therefore, be considered to be in an optimal dose window, allowing for efficient GVHD prevention and a low number of related infections and relapses, as previously reported.20
Our study does have several obvious biases: it was a retrospective, non-randomized study, and molecular markers such as NPM1 and FLT3-ITD were not available for all patients, which made it difficult to analyze the prognosis impact of those markers in our cohort. As it was not a prospective, randomized study, the choice of the conditioning regimen was based on physicians’ appraisal, which limits the assessment of the impact of different individual conditioning regimens. In addition, a busulfan pharmacokinetics analysis would be of great interest and could help to determine the effect of busulfan dose in terms of both disease control and transplant-related morbidity and mortality. However, the strength of our results lies in the homogeneity of the cohort, with regards to disease type and transplant characteristics, which makes our conclusions more robust. Only two transplantation centers were included in the study, which led to homogeneous supportive care and evaluation of the patients.
We conclude that a conditioning regimen including fludarabine, busulfan, and rabbit anti-thymoglobulin (5 mg/kg) is well tolerated and can lead to long-term disease control. Higher busulfan doses were not associated with greater toxicity. Such a regimen may be effective for tuning the dose intensity by modulating the busulfan dose according to disease and toxicity risks. The impact of dose-intensity on post-transplant outcome remains a central issue and only a randomized, prospective study would be able to provide a final answer to this important question. A randomized multicenter study of this nature is now underway in France (Study AAA-IPC 2°11-003, EUDRACT number: 2013-001935-36) and aims to compare different doses of busulfan in a common conditioning platform based on fludarabine, three different doses of busulfan (260 mg/m2, 390 mg/m2, and 520 mg/m2), and rabbit anti-thymoglobulin (5 mg/kg). To date, a few patients have been randomized into this large, prospective study, which will help to determine whether higher doses of busulfan could lower relapse risk without increasing transplant-related toxicity.
Acknowledgments
The authors would like to thank the nursing staff, under the supervision of Laurence Caymaris, Head Nurse, for providing excellent care for the patients, and the physicians of the Hematology Department at the Paoli-Calmettes Institute for their important study contributions and dedicated care of patients.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–9. [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Lazarus HM, Keating A. Myeloablative conditioning regimens for AML allografts: 30 years later. Bone Marrow Transplant. 2003;32(10):969–78. [DOI] [PubMed] [Google Scholar]
- 3.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368(9550):1894–907. [DOI] [PubMed] [Google Scholar]
- 4.Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(27):4570–7. [DOI] [PubMed] [Google Scholar]
- 5.Sayer HG, Kroger M, Beyer J, Kiehl M, Klein SA, Schaefer-Eckart K, et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31(12):1089–95. [DOI] [PubMed] [Google Scholar]
- 6.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24(3):444–53. [DOI] [PubMed] [Google Scholar]
- 7.Blaise DP, Michel Boiron J, Faucher C, Mohty M, Bay JO, Bardoux VJ, et al. Reduced intensity conditioning prior to allogeneic stem cell transplantation for patients with acute myeloblastic leukemia as a first-line treatment. Cancer. 2005;104(9):1931–8. [DOI] [PubMed] [Google Scholar]
- 8.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–400. [DOI] [PubMed] [Google Scholar]
- 9.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12(10): 1047–55. [DOI] [PubMed] [Google Scholar]
- 10.Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martino R, de Wreede L, Fiocco M, van Biezen A, von dem Borne PA, Hamladji RM, et al. Comparison of conditioning regimens of various intensities for allogeneic hematopoietic SCT using HLA-identical sibling donors in AML and MDS with <10% BM blasts: a report from EBMT. Bone Marrow Transplant. 2013;48(6):761–70. [DOI] [PubMed] [Google Scholar]
- 12.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104(3):865–72. [DOI] [PubMed] [Google Scholar]
- 13.Blaise D, Maraninchi D, Michallet M, Reiffers J, Jouet JP, Milpied N, et al. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving HLA-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97(11): 3669–71. [DOI] [PubMed] [Google Scholar]
- 14.Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79(10):2578–82. [PubMed] [Google Scholar]
- 15.Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20(2):322–8. [DOI] [PubMed] [Google Scholar]
- 16.Gorin NC, Labopin M, Boiron JM, Theorin N, Littlewood T, Slavin S, et al. Results of genoidentical hemopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond–the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24(24):3959–66. [DOI] [PubMed] [Google Scholar]
- 17.van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23(24):5728–38. [DOI] [PubMed] [Google Scholar]
- 18.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28(17):2859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson BS, de Lima M, Thall PF, Wang X, Couriel D, Korbling M, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6): 672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell JA, Duan Q, Chaudhry MA, Savoie ML, Balogh A, Turner AR, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008;14(8): 888–95. [DOI] [PubMed] [Google Scholar]
- 21.Iravani M, Evazi MR, Mousavi SA, Shamshiri AR, Tavakoli M, Ashouri A, et al. Fludarabine and busulfan as a myeloablative conditioning regimen for allogeneic stem cell transplantation in high- and standard-risk leukemic patients. Bone Marrow Transplant. 2007;40(2):105–10. [DOI] [PubMed] [Google Scholar]
- 22.Blaise D, Farnault L, Faucher C, Marchetti N, Furst S, El Cheikh J, et al. Reduced-intensity conditioning with fludarabin, oral busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol. 2010;38(12):1241–50. [DOI] [PubMed] [Google Scholar]
- 23.Devillier R, Crocchiolo R, Castagna L, Furst S, El Cheikh J, Faucher C, et al. The increase from 2.5 to 5 mg/kg of rabbit anti-thymocyte-globulin dose in reduced intensity conditioning reduces acute and chronic GVHD for patients with myeloid malignancies undergoing allo-SCT. Bone Marrow Transplant. 2012;47(5):639–45. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Sandmaier BM, Storer BE, Maris MB, Baron F, Maloney DG, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246–54. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. [DOI] [PubMed] [Google Scholar]
- 27.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74. [DOI] [PubMed] [Google Scholar]
- 28.Blaise D, Castagna L. Do different conditioning regimens really make a difference? Am Soc Hematol Educ Program, 2012:237–45. [DOI] [PubMed] [Google Scholar]
- 29.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 30.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 32.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 33.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102(2):470–6. [DOI] [PubMed] [Google Scholar]
- 34.Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17(10):1490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laport GG, Sandmaier BM, Storer BE, Scott BL, Stuart MJ, Lange T, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European Group for Blood and Marrow Transplantation (EBMT). Leukemia. 2005;19(12):2304–12. [DOI] [PubMed] [Google Scholar]
- 37.Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Leukemia. 2012;26(12):2462–8. [DOI] [PubMed] [Google Scholar]
- 38.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005;19(6): 916–20. [DOI] [PubMed] [Google Scholar]
- 40.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23(36):9387–93. [DOI] [PubMed] [Google Scholar]
- 41.Wallen H, Gooley TA, Deeg HJ, Pagel JM, Press OW, Appelbaum FR, et al. Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439–46. [DOI] [PubMed] [Google Scholar]
- 42.Bacigalupo A. Antithymocyte globulin for prevention of graft-versus-host disease. Curr Opin Hematol. 2005;12(6):457–62. [DOI] [PubMed] [Google Scholar]
- 43.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. [DOI] [PubMed] [Google Scholar]
- 44.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–94. [DOI] [PubMed] [Google Scholar]
- 45.Peric Z, Cahu X, Chevallier P, Brissot E, Malard F, Guillaume T, et al. Features of Epstein-Barr virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25(6):932–8. [DOI] [PubMed] [Google Scholar]


