Figure 4.
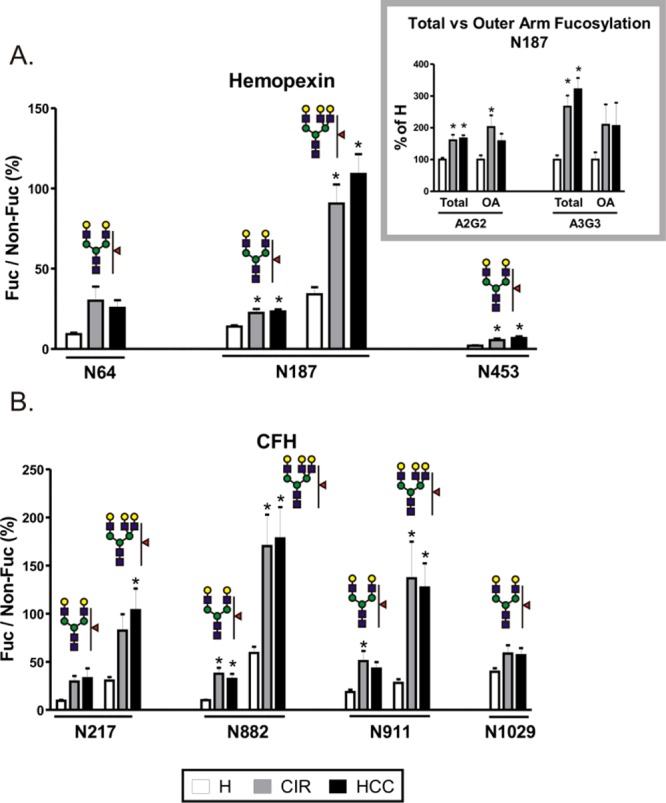
Fucosylation of desialylated glycopetides in individual samples of healthy controls (H), cirrhosis (CIR), and HCC patients. Tryptic glycopeptides of (A) hemopexin and (B) CFH from the heme-bound fraction of individual patient samples were quantified by LC-MS MRM. Data are expressed as a relative ratio of signal intensities of fucosylated glycopeptide to its nonfucosylated counterpart monitored as the 366 transition (Hex–HexNAc) and shown as percent of the nonfucosylated form. Glycan structures representing specific glycoforms are indicated above each group of corresponding bars; the position of the glycosylation site in the protein sequence is shown below. Inset. Comparison of total and outer arm fucosylation at the N187 site of hemopexin. Outer arm fucosylation was quantified as the 512 transition (Fuc–GlcNAc–Gal) of fucosylated precursor normalized to the 366 transition of its nonfucosylated counterpart; total fucosylation was quantified as above. The relative change in fucosylation in liver disease groups is shown as a percent of H. OA; outer arm fucosylation. Results are shown as mean ± SEM; ∗, P < 0.05 vs H.
