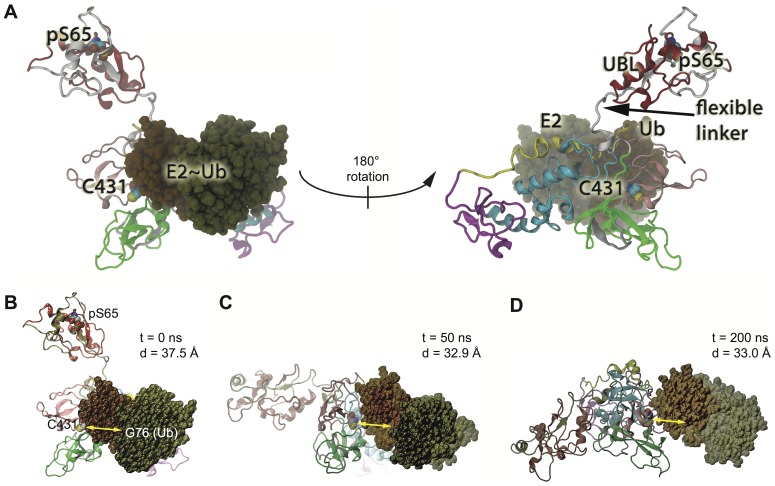
Figure 6. Protein-protein docking for Parkin and a charged E2∼Ub complex.
A) Ten conformations spanning closed Parkin to fully opened Parkin (see Figure 4) were sampled for protein-protein interactions. The E2-Ub complex was docked at the midpoint UBL position (state 2/3) when the REP region liberated the binding site in RING1 (Figure 4B/C). This conformation showed fewer steric clashes and lowest energy profile. The docking in the same position is shown and rotated 180° to reveal the other side. Residues of the Ubch5a-Ub complex are indicated by color (dark green and brown, respectively). B–D) Docking at the RING1 interface is critical for E2-Ub progression towards the active site of Parkin. E2 binding at RING1 limits the UBL-linker mobility preventing the drift back to the original, auto-inhibited state. B) Same orientation as in A (left side) predicted as an optimal docking conformation after 0 ns of MDS. The distance between Gly76 of Ub and Parkin's active site (Cys431) is indicated. C) Following unbiased MD (>200 ns), the E2-Ub complex moves towards the Parkin's active center. The decreased distance is shown after 50 ns. D) Overall re-orientation of the UBL domain and the E2-Ub complex is shown after 200 ns.