Figure 3.
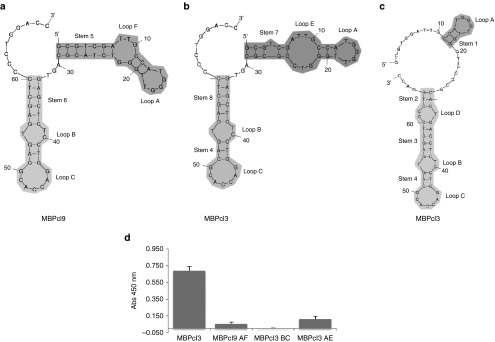
Aptamer conformations and their binding activities. Upper panel shows schematic representations of secondary structures of MBPcl3 and MBPcl9 as predicted by the mfold program. (a) is the predicted most favorable structure for MBPcl9; (b) and (c) are two different possible conformations for MBPcl3 with similar free energies. The myelin basic protein (MBP)-binding activity of three different MBPcl3 domains was determined by an enzyme-linked immunosorbent assay (ELISA)-like assay (d). 0.5 µg of pure MBP was immobilized onto wells of a microtiter plate and incubated with MBPcl3 or equal nmol of MBPcl3 or MBPcl9 truncated products. Truncation was performed to retain a stem-loop structure. MBPcl9 AF includes Stem 5, Loop A and F; MBPcl3 BC includes stem 4 and 8 and loops B and C; MBPcl3 AE includes stem 7 with loops A and E. Experiments were performed in duplicate. The asterisks indicate a significant difference in comparison with MBPcl3 (P < 0.05, Student's t-test). Data are shown as mean ± SD.
