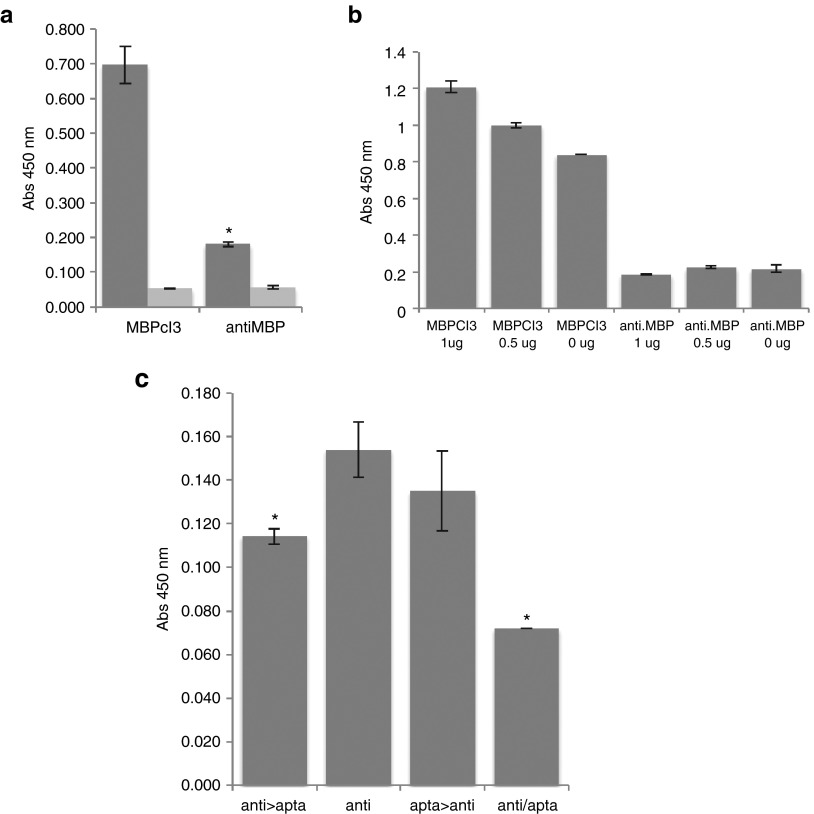
Figure 5.
The myelin basic protein (MBP)-binding activity of an antibody compared to MBPcl3. (a) Equal nmol of MBPcl3 and of the antibody were incubated with the MBP that was first immobilized on a microtiter plate. Wells without MBP were used as controls (light gray). (b) A cleared lysate of Hela cells was immobilized on a microtiter plate containing 1 or 0.5 µg of pure MBP. Wells were then blocked with 3% BSA and after washing 7 pmol of aptamer or antibody was added. (c) 0.5 µg of pure MBP was immobilized onto a microtiter plate and incubated with 0.028 nmol of MBPcl3, 0.010 nmol of anti-MBP antibody or with both at different times. Columns from left to right: (1) MBP incubated with anti-MBP antibody, washed and then with the MBPcl3 aptamer. (2) MBP incubated with the antibody only. (3) MBP incubated with MBPcl3, washed and then with anti-MBP antibody. (4) anti-MBP and MBPcl3 aptamer incubated together. For (a), (b), and (c), fraction of bound antibody was detected with an horseradish peroxidase (HRP)-conjugated anti-rabbit antibody and developed with 3,3',5,5'-tetramethylbenzidine (TMB) substrate, while for (a) and (b), fraction of bound aptamer was detected with an HRP-conjugated avidin. Experiments were performed in triplicate. The asterisks indicate a significant difference in comparison with MBPcl3 (a) or with “anti” (b) (P < 0.05, Student's t-test). Data are shown as mean ± SD.