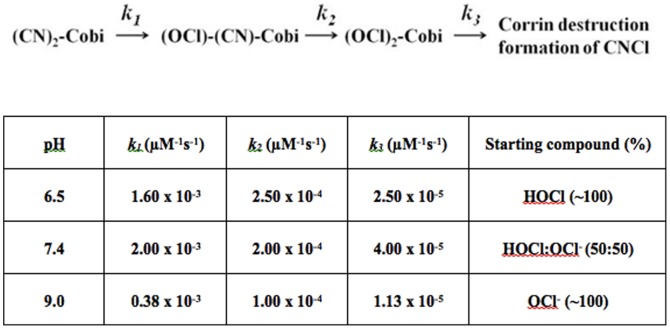
Figure 7. Rate constants for the formation and decay of the intermediates that formed upon mixing (CN)2-Cbi with HOCl.
(CN)2-Cbi was rapid-mixed with buffer containing HOCl at various concentrations. Rates of complex formation and decay were determined at three different pHs (6.5, 7.4, and 9.0) by following absorbance change at 613 or 493 nm, at 10°C, and the rate constants determined as described in the text. These data are representative of three independent experiments and the standard error for each individual rate constant has been estimated to be less than 3%.