Abstract
We have shown that some patients presenting with chronic bronchodilator-resistant non-productive cough have a global atopic tendency and cough hypersensitivity without nonspecific bronchial hyperresponsiveness, abbreviated as atopic cough (AC). The cough can be treated successfully with histamine H1 antagonists and/or glucocorticoids. Eosinophilic tracheobronchitis and cough hypersensitivity are pathological and physiological characteristics of AC. Fungus-associated chronic cough (FACC) is defined as chronic cough associated with basidiomycetous (BM) fungi found in induced sputum, and recognition of FACC has provided the possibility of using antifungal drugs as new treatment strategies. Bjerkandera adusta is a wood decay BM fungus, which has attracted attention because of its potential role in enhancing the severity of cough symptoms in FACC patients by sensitization to this fungus. Before making a diagnosis of “idiopathic cough” in cases of chronic refractory cough, remaining intractable cough-related laryngeal sensations, such as “a sensation of mucus in the throat (SMIT),” which is correlated with fungal colonization, should be evaluated and treated appropriately in each patient. The new findings, i.e., the detection of environmental mushroom spores that should not be present in the human airways in addition to the good clinical response of patients to antifungal drugs, may lead to the development of novel strategies for treatment of chronic cough.
Keywords: Atopic cough (AC), cough variant asthma (CVA), fungus-associated chronic cough (FACC), Bjerkandera adusta, cough-related laryngeal sensations
Chronic cough that cannot be managed after basic evaluation is a common reason for referral to respiratory outpatient clinics (1). Despite extensive diagnostic evaluation and numerous treatment guidelines (2-5), a diagnosis cannot be made in a subgroup of chronic cough patients. This condition is termed by chronic idiopathic cough (CIC) (6,7), which is difficult to treat by any current treatment strategy. Therefore, there is a great deal of research interest regarding the identification of novel antitussive drugs (8,9) and the establishment of novel therapeutic strategies for such cases. Here, we describe the contribution of our series of studies concerning atopic cough (AC) (10) and fungal allergy in unexplained chronic cough (11,12).
Atopic cough (AC)
We have shown that some patients presenting with chronic bronchodilator-resistant non-productive cough have global atopic tendency and airway cough hypersensitivity without nonspecific bronchial hyperresponsiveness, abbreviated as AC. The cough can be treated successfully with histamine H1 antagonists and/or glucocorticoids (10).
Investigations of pathological findings of AC
We prospectively investigated the location and intensity of eosinophil infiltration using tracheal and bronchial biopsy specimens and bronchoalveolar lavage fluid (BALF) in patients with AC. Although the major site of eosinophilic infiltration of cough variant asthma (CVA) (13) is the peripheral airway, that of AC is assumed to be the central airway, especially the trachea.
The main site of complaints in most patients with AC, “a sensation of irritation in the throat (SIT),” is around the trachea in front of the neck region. Therefore, we planned to obtain tracheal mucosa of AC patients. To prevent unexpected slipping during biopsy of the tracheal wall, we performed tracheal biopsy with pushing the needle of cup forceps into the tracheal wall. We gradually grasped the wall material, and successfully obtained the tracheal wall specimen.
The macroscopic specimen of the tracheal mucosa showed eosinophilic tracheitis in AC patients. In AC patients, we found eosinophil infiltration in the tracheal wall, and there was a significant difference in the number of eosinophils between AC patients and healthy control subjects. A similar difference was also seen in the bronchial wall.
In contrast, the results of BALF reflect the peripheral airways. Bronchoalveolar lavage (BAL) eosinophilia was not seen in AC patients.
Taken together, these observations suggest that the major sites of eosinophilic infiltration are the central airway, including the trachea in AC, and central to peripheral airway in CVA, similar to bronchial asthma (BA).
Investigation of physiological findings of AC
In AC patients, cough threshold was heightened at first visit, and it became normalized after treatment, when cough was relieved. In contrast, in CVA patients, cough threshold was within the normal range as a whole at first visit. After treatment, the cough threshold did not change significantly, suggesting that the airway cough receptors have no role in the pathophysiology of CVA (14).
Our series of studies have demonstrated that eosinophilic tracheobronchitis and cough hypersensitivity are pathological and physiological characteristics of AC.
Clinical features of AC
The clinical features of AC are as follows: (I) chronic bronchodilator-resistant nonproductive cough with “tickle” in the throat and/or “a SIT” lasting for more than eight weeks; (II) absence of wheezing, dyspnea, hemoptysis, or pleurisy, and no adventitious lung sounds on physical examination; (III) presence of one or more global atopic findings, including past history and/or complication of allergic diseases except BA, family history of allergic diseases, peripheral blood eosinophilia, elevated total IgE level in the serum, positive specific IgE antibody to common aeroallergens, and positive allergen skin test; (IV) presence of eosinophils in hypertonic saline-induced sputum and/or submucosa of biopsied trachea and/or bronchi; (V) normal limits of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio; (VI) no bronchial reversibility defined as less than a 5% increase in FEV1 after inhalation of 300 µg salbutamol following 250 mg aminophylline injection; (VII) bronchial responsiveness within normal limits; (VIII) increased airway cough reflex sensitivity; and (IX) complete relief of the cough upon treatment with histamine H1 antagonists, inhaled corticosteroid (ICS) therapy, and/or oral corticosteroid therapy.
Notable differences between AC and CVA
A clinical feature of CVA is a good response to bronchodilator therapy, which is in contrast to AC (10). We measured cough response to methacholine (Mch)-induced bronchoconstriction in patients with CVA, typical asthma (BA), and in normal healthy volunteers (15,16). The degree of induced bronchoconstriction was assessed using partial and full flow-volume curves. The number of coughs induced by Mch inhalation was counted during and for 30 min after Mch inhalation when PEF40 (15) was decreased by 35% (defined as mild bronchoconstriction) and FEV1 was reduced by 20% (defined as more severe bronchoconstriction). Although coughs were hardly provoked in normal subjects, many coughs were provoked in patients with CVA, at mild bronchoconstriction. In contrast with CVA, bronchoconstriction-triggered cough was impaired in typical asthma. Furthermore, cough response to bronchoconstriction induced by Mch in AC patients was the same as that in normal subjects (unpublished data). It was concluded that heightened cough response to bronchoconstriction is a fundamental feature of CVA, which is different from BA. Thus, CVA is not merely a clinical entity represented as a mild type of asthma.
Our previous study demonstrated that exhaled NO levels in patients with AC were significantly lower than those in patients with CVA and BA. There was no significant difference in exhaled NO level between patients with CVA and BA. Exhaled NO may reflect eosinophilic inflammation of peripheral airways and its measurement may be useful in differentiating CVA from AC and other causes of chronic nonproductive cough (17).
Possible peripheral cough response pathway
We postulated that there may be a peripheral cough response pathway located in and around airway smooth muscles (Figure 1). The A line from the epithelium of the superficial bronchi to the cough center consists with afferent Aδ fibers or C fibers, and the B line from the smooth muscle to the cough center consists of afferent Aδ fibers. Hyperstimulation of the A line occurs as a result of stimuli with sputum production or endobronchial foreign body exposure, and increased response of the A line (cough reflex hypersensitivity) has been demonstrated in AC, gastroesophageal reflux-associated cough (GER-cough) (18), and ACE inhibitor-induced cough. Hyperstimulation of the B line is a possible mechanism of cough in BA. Increased response in the B line is the fundamental mechanism of cough in CVA.
Figure 1.
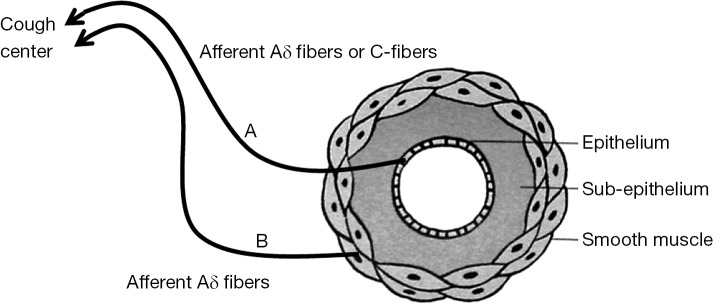
Possible peripheral cough response pathway. A, hyperstimulation: productive cough, endobronchial foreign body; increased response (cough reflex hypersensitivity): atopic cough, GERD, ACE-I-induced cough; B, kyperstimulation: bronchial asthma; increased response: cough variant asthma.
It is strongly recommended that definite diagnosis of chronic cough should be made based not on the efficacy of medical treatments but on the pathophysiological features.
Prognosis of AC and CVA
Onset of typical asthma occurred in only one (1.2%) of the patients with AC. In patients with CVA, typical asthma developed in two of 35 (5.7%) patients taking BDP and six of 20 (30%) untreated patients (P<0.02). These findings suggest that CVA is a precursor of typical asthma, while AC is not. Treatment with ICS may prevent the transformation of CVA into typical asthma (19).
Spectrum and frequency of causes of chronic cough in the Hokuriku area of Japan
A prospective multicenter study conducted in the Hokuriku area of Japan (20) showed the three most common causes of chronic cough to be AC (35.8%), CVA (43.6%), and sinobronchial syndrome (SBS) (21) (25.5%). The frequency of GERD was not high (2.4%), as reported in the Western countries (18).
Proposal for managing AC and CVA (Table 1)
Table 1. Proposal for managing atopic cough and cough variant asthma.
| Atopic cough | Cough variant asthma | |
|---|---|---|
| Reliever | ||
| The first-line agents | Histamine H1 antagonists | Rapid-acting bronchodilators |
| The second-line agent | ICS (large particle size) | Theophylline, LTRA, ICS (small particle size) |
| Controller | None | ICS (small particle size) |
ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist.
Although the fundamental conditions of AC and CVA are involved in the same spectrum of eosinophilic airway disorders, the major sites of each disease are different, and therefore their responses to bronchodilator therapy differ.
The first-line agents for treatment of AC (central eosinophilic airway disease) and CVA (peripheral eosinophilic airway disease) are histamine H1 antagonists and rapid-acting bronchodilators, respectively. ICS is not appropriate as a first-line agent for treatment of CVA. The second-line agent for AC is ICS (large particle size) and those for CVA are theophylline, leukotriene receptor antagonists, and ICS (small particle size). In contrast, although ICS is not necessary for control of AC, ICS (small particle size) is recommended for control of CVA.
Thus, ICS therapies against AC or CVA should be selected both with regard to usage and adequate particle size. Therefore, AC and CVA should be distinguished as strictly as possible.
Fungal allergy
There have been many attempts to detect fungi in bronchial specimens from patients with allergic fungal respiratory diseases (22), such as allergic bronchopulmonary mycosis (ABPM) (23), eosinophilic pneumonia (24), and hypersensitive pneumonitis (25), and with the increasing interest in the role of fungal sensitization in BA (26) such studies have spread to encompass not only BA patients (27-30) but also those with chronic cough.
Chronic cough and environmental fungi
We have previously reported cases of AC caused by a hypersensitivity to Trichosporon asahii (31), Pichia guilliermondii (32), Streptomyces albus (33), and also reported the first case of nonasthmatic sputum eosinophilia caused by allergic reaction to a basidiomycetous (BM) fungus in which the increase of eosinophils in the induced sputum was established by repeated environmental surveys to be closely related to the appearance of BM fungi in the patient’s house (34).
Initially we performed pharyngeal swab cultures for the detection of fungi in 141 patients with chronic nonproductive cough and identified Candida and BM in 10.6% and 6.4% of all the examined patients, respectively (35). Since BM fungi are rarely detected in the culture of pharyngeal swabs taken from non-coughers, and the positive ratio of the immediate subcutaneous reaction for BM fungi in allergic airway diseases such as AC, CVA, and cough-predominant asthma was significantly higher than that in non-coughers (unpublished data), we suspected that the positive culture results were not caused by an environmental fungal contamination.
Gargling with amphotericin B was efficatious for AC patient whose pharyngeal swab yielded BM fungus (36). Because the patient was resistant to previously established treatments, these clinical experiences were surprising. Thus we started the series of research to clarify the clinical entity of chronic cough patients responsive to antifungal drugs (37).
Fungus-associated chronic cough (FACC)
FACC was introduced as a new chronic cough condition together with obstructive sleep apnea and chronic tonsillar enlargement (11,12). FACC (38) is defined as chronic cough associated with BM fungi found in induced sputum, and recognition of FACC has provided the possibility of the using antifungal drugs as new treatment strategies (39). Therefore, it is recommended to detect BM fungi in bronchial specimens from chronic cough patients (35). In the previous study, BM fungi were detected in sputum samples from 39 (22.8%) of 171 chronic cough patients. Even though there may be geographical variations, such FACC patients with a sensation of mucus in the throat (SMIT) (40) may have been overlooked among chronic cough patients in ordinary clinics.
We assume that there are three types of FACC: (I) sole colonization with BM fungi (pure-FACC); (II) sensitization with BM fungi (allergic fungal cough, AFC) (41); and (III) colonization and/or sensitization by BM fungi in addition to established chronic cough, such as CVA (13), AC (10), upper airway cough syndrome (1), gastro-esophageal reflux disease (18), and cough hypersensitivity syndrome (42). Therefore, FACC should be recognized from the viewpoint of FACC component which is often correlated with SMIT.
The importance of BM fungi cultured from the sputum of chronic idiopathic cough (CIC)
CIC (6) has been reported as a subgroup of chronic coughers in whom a diagnosis cannot be made even after thorough systematic investigation. Even though the major causes of chronic cough exhibit geographical variation, it is important to investigate how to manage chronic intractable cough and how to address the problem of CIC and minimize the diagnosis of CIC.
The positive ratio of BM cultured from the sputa of CIC patients (62.5%) was significantly (P=0.0061) higher than that of non-CIC patients (16.7%). The existence of BM fungi in induced sputum may be an important factor for distinguishing the clinical manifestation of CIC from that of non-CIC (43). The clinical approach from the aspect of fungal allergy may serve as a clue that may aid in the successful management of CIC.
Recognition of environmental mushroom spores may alter our outlook regarding chronic cough
The Basidiomycota (44) consist of a number of species, such as Trichosporon spp., Cryptococcus spp., and other mushroom spores. That is, environmental fungi that are closely associated to chronic cough are mushroom spores that grow mostly in fields. It is necessary to find and identify the peculiar mushrooms that are correlated with allergic respiratory disorders (45,46).
It should be noted that although it requires only 2 days to detect the common fungal allergen Aspergillus in cultured sputum samples, a period of approximately 10 days is required to culture white colonies of mushroom spores on either potato dextrose agar or Sabouraud’s dextrose agar containing chloramphenicol (39).
In the slide culture method (30 °C for 2-3 weeks), fungi have been identified mainly based on the morphological features of the conidial head (conidia). Therefore, filamentous BM fungi that do not have conidia or fruiting bodies were not identified, and such filamentous fungi may have been classified as belonging to either the category of “mycelia, hyphe” or “unknown”.
Based on further studies using 28S rDNA (D1/D2) sequencing and analysis, we postulated that Bjerkandera adusta (GenBank sequence AB096738) may be an important potential etiological agent in chronic cough (41).
PCR and sequencing
PCR was performed with the primers for the 28 SrDNA partial sequence (D1/D2 region) (47), NL-1 (5'-GCATATCAATAAGCGGAGGAAAAG-3') and NL-4 (5'-GGTCCGTGTTTCAAGACGG-3'), under the following conditions: 25 cycles of 94 °C, 1 min; 60 °C, 15 s; and 72 °C, 15 s. The products were detected as a single band of 0.5 kbp by agarose gel analysis. The products were detected as a single band of 0.5 kbp by agarose gel analysis. Both strands of the PCR products were directly sequenced using a DNA sequencing kit (Applied Biosystems) and an automatic sequencer (Genetic Analyzer 310; Applied Biosystems) according to the manufacturer’s instructions. The DDBJ/EMBL/GenBank nucleotide sequence databases were searched for matches of the confirmed sequences by using BLAST programs (48).
Allergic fungal cough (AFC)
Preparation of the antigenic solution
One liter of Sabouraud’s dextrose broth in 3 liter flasks was sterilized by autoclaving at 121 °C for 20 min. A total of 5 mL of B. adusta (NBRC 4983) spore suspension (105 spores per mL) in sterile physiological saline from 14 day-old Sabouraud’s dextrose agar culture were used to inoculate the flask. The flasks were shaken at 25 °C at 150 rpm in a rotary shaker incubator. The supernatant was then dialyzed against 5 mM ammonium bicarbonate and lyophilized.
Immediate-type skin test and serological test
Reactions to the Bjerkandera adusta antigen
The antigenic solution (polysaccharide) was injected intradermally with a tuberculin syringe (0.02 mL, 1 mg/mL) to assess the skin response to the solution. The result was judged to be positive in a case of the longer axis of the flare beyond 10 mm at 15 minutes after the injection.
Bronchoprovocation test
When cough symptom improved after the standard therapy, patients were subjected to bronchoprovocation test using the antigenic solution (49); B. adusta and Penicillium thomii (as control) (2 mL of culture-filtrate antigen, 1 mg/mL) through a jet nebulizer. The responses were assessed to be positive when laboratory findings such as WBC and CRP elevated significantly and/or patients developed cough attacks with a significant increase in cough reflex sensitivity to inhaled capsaicin which was measured before and 24 hours after the provocation test.
Eight patients with FACC whose sputum yielded B. adusta and who were sensitized to the fungus were diagnosed to have AFC caused by B. adusta (41). Further study demonstrated that B. adusta was one of the environmental fungi which attracted attention because of its potential role in enhancing severity of cough symptom in FACC patients by sensitization to this fungus.
The clinical features of AFC are (I) chronic intractable cough with a SIT and/or a SMIT, which lasted for more than 8 weeks; (II) absence of wheezing, dyspnea, hemoptysis, or pleurisy, and no adventitious lung sounds on physical examination; (III) the presence of environmental fungi such as BM fungus, particularly, B. adusta; (IV) at least one of the positive reactions to immediate cutaneous reactivity and/or inhalation bronchoprovocation test and/or lymphocyte stimulation test to the fungus; (V) good clinical response to antifungal drugs for which a considerable period of time (more than 10 weeks approximately) is required for the complete remission of the cough symptoms; (VI) frequent recurrence of cough; (VII) peripheral blood eosinophilia, elevated total IgE level in the serum, and eosinophilia in the induced sputum, which are not necessarily observable; (VIII) normal FEV1, FVC, and FEV1/FVC ratios; (IX) normal to slightly increased airway cough reflex sensitivity; (X) bronchial responsiveness within normal limits.
Characteristics and prevalence of Bjerkandera adusta
B. adusta is a wood decay BM fungus (50,51), which has attracted attention because of its potential role in enhancing the severity of cough symptoms in FACC patients by sensitization to this fungus. Further studies demonstrated growth of filamentous B. adusta at 4-37 °C on Sabouraud dextrose agar and showed that abundant asexual spores were produced from the hyphae of this fungus (52). Such a peculiar characteristic of B. adusta, among the approximately 31,000 species in the BM phylum, may enable this fungus to colonize the human bronchi. Studies focusing on the role of fungal colonization in sensitization of AFC patients demonstrated that colonization by B. adusta is necessary in the process of sensitization to this fungus (53).
Therefore, antifungal drugs have provided a new treatment strategy for chronic intractable cough in reducing or eradicating the colonizing antigen. Our recent study demonstrated the possibility of predicting positive results for sensitization to B. adusta in the efficacy of antifungal therapy in CIC patients (54).
Sautour et al. (55) reported that in outdoor samples, B. adusta (8%) was the third most frequent species, especially in summer, and was the third and fourth most common species in the adult hematology unit (13%) and the pediatric hematology unit (11%), respectively, at a French hospital. They also mentioned that the concentration of this fungus was particularly high during the winter 2006/07, with a percentage close to 30% in indoor samples, suggesting that AFC may be recognized outside Japan in the near future. As B. adusta, a mushroom spore with a worldwide distribution may have potential to enhance the clinical manifestations of allergic bronchial disorders, natural phenomena such as yellow sand dust may influence human health on a worldwide scale (56,57).
Antifungal drugs against fungal colonization
By focusing on the role of fungal colonization in sensitization of patients with AFC, it has recently been demonstrated that colonization by B. adusta is necessary in the process of sensitization to this fungus. Although the routine use of antifungal drugs against fungus-sensitized asthma or severe asthma with fungus sensitization (SAFS) (58) requires further evaluation (26), in such cases of FACC, antifungal therapy is expected to have advantages for reducing or eradicating the colonizing antigen and thus preventing the sensitization process.
Gonzalez et al. (59) reported that B. adusta was susceptible to itraconazole (ITCZ), amphotericin B, and voriconazole in vitro, with MIC values of 0.125, 0.25, and 0.5 µg/mL, respectively. Therefore, antifungal therapy is expected to have advantages for reducing or eradicating the colonizing fungal spores in the airway of chronic cough patients (60).
Clinical experience with low-dose itraconazole (ITCZ) in CIC
There is a great deal of research interest regarding the identification of novel antitussive drugs (61) and the establishment of novel therapeutic strategies.
The presence of BM fungi in induced sputum is an important clinical finding in CIC (6). However, the efficacy of anti-fungal therapy for CIC has not been evaluated. In our recent study, we selected ten patients with CIC and carried out allergological examinations for B. adusta, a BM fungus that has been shown to enhance cough severity (54). The efficacy of low-dose ITCZ therapy (50 mg/day) for 14 days as an adjunctive therapy was estimated with use of cough visual analog scale (VAS) and the Japanese version of the Leicester Cough Questionnaire (J-LCQ) (62,63). We evaluated whether there was a recognizable clinical or allergological pattern that could predict the efficacy of ITCZ therapy in CIC patients.
At present, it is not possible to conclude whether ITCZ therapy provides sufficient relief of cough in CIC patients. However, a peculiar laryngeal sensation of CIC patients, represented as SMIT (40), almost disappeared with ITCZ therapy.
Chronic cough management: dealing with a sensation of mucus in the throat (SMIT) and a sensation of irritation in the throat (SIT)
Among the various types of laryngeal paresthesia suffered by chronic cough patients (64), we have often encountered “SIT” in clinical practice. Our study demonstrated that there was a significant difference in capsaicin cough sensitivity between SIT-positive and SIT-negative groups (65). Although the mechanism of cough hypersensitivity should be investigated in each chronic cough patient, the establishment of treatment strategies for SIT would have advantages for treating chronic cough patients in ordinary clinics.
Suppression of eosinophilic inflammation in the central airway is considered to be efficacious in such cases, i.e., administration of ICS (large particle size). It is not recommended to make a diagnosis of chronic cough based merely on the presence of SIT. The mechanism of cough hypersensitivity should be investigated in each chronic cough patient with SIT.
These results suggested that among the various types of laryngeal paresthesia, SMIT alone can predict the presence of fungal colonization in chronic cough patients. Although SMIT should be treated together with the cough itself, it is usually overlooked because SMIT is not reflected by physiological examinations or cough-specific questionnaires, and therefore it has not been treated adequately in the cough clinic. We propose that next to “cough” or “sputum,” SMIT may become a key clinical symptom for predicting the efficacy of antifungal therapy in chronic cough patients.
Total management of chronic refractory cough patients
For the successful total management of chronic cough, we propose the following two steps.
Detection of asthmatic component
It is an initial problem to find the asthmatic component with use of rapid-acting bronchodilator as a first-line relieving therapy of CVA. To relieve more severe symptoms of CVA, leukotriene antagonists, theophylline, and ICS would be prescribed as second-line therapy. Furthermore, continuous ICS is recommended for preventing the progression of CVA into BA.
Selection of additional therapies for coexisting cough-related laryngeal sensations
Chronic cough patients suffer from various types of laryngeal paresthesia (66). Some local laryngeal sensations can be relieved by use of previously established therapies: (I) SIT, tickle in the throat (as an AC component), histamine H1 antagonists; (II) a sensation of catarrh down the throat (as SBS component), low-dose macrolides (21); (III) a SMIT (as FACC component), low-dose ITCZ; (IV) throat clearing, hoarseness (as GER component), PPI (18); and (V) urge to cough or others, central sensitization suppressants, cough suppressant therapy (67).
Before making a diagnosis of “idiopathic cough” in cases of chronic refractory cough, remaining intractable cough-related laryngeal sensations should be evaluated and treated appropriately in each patient (68).
Chronic cough in a tsunami-affected town
We suspected that environmental fungi were present in the air because the collapse of houses may affect the respiratory conditions of local residents rather than asbestos. In fact, although previously arriving medical teams prescribed various medications (such as clarithromycin, antitussive drugs, histamine H1 antagonists, ICS, bronchodilators, and proton-pomp inhibitors) during this period, the cough symptoms of these patients had not yet subsided. A low dose of ITCZ therapy (50 mg/day for 14 days) showed excellent efficacy for the respiratory symptoms in these cases. The outbreak of respiratory disorder in the refuge has subsided. Our clinical experience indicated that the new clinical concept of FACC has some advantages in managing unexplained cough in a tsunami-affected town (69).
Summary
Our series of studies have demonstrated that eosinophilic tracheobronchitis and cough hypersensitivity are pathological and physiological characteristics of AC, and heightened cough response to bronchoconstriction is a fundamental physiological feature of CVA.
FACC is defined as chronic cough associated with BM fungi found in induced sputum, and recognition of FACC has provided the possibility of using antifungal drugs as new treatment strategies. B.adusta is a wood decay BM fungus that has attracted attention because of its potential role in enhancing the severity of cough symptoms in FACC patients by sensitization to this fungus.
Before making a diagnosis of “idiopathic cough” in cases of chronic refractory cough, remaining intractable cough-related laryngeal sensations such as “a SIT” or “a SMIT” should be evaluated and treated appropriately in each patient.
The new findings, i.e., the detection of environmental mushroom spores that should not be present in the human airways in addition to the good clinical response of patients to antifungal drugs, may lead to the development of novel strategies for treatment of chronic cough. Information regarding the presence of mushroom spores in the sputum of chronic cough patients may alter the ways in which we approach clinical research in this field, and may have advantages with regard to patient benefits, including improvements in quality of life and reduction of medical expenses.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990;141:640-7. [DOI] [PubMed] [Google Scholar]
- 2.Fujimura M, Uchida Y, Niimi A. eds. Guidelines for diagnosis and treatment of chronic cough, 2nd ed. Tokyo: Kita Media, 2003:1-35. [Google Scholar]
- 3.Committee for the Japanese Respiratory Society Guidelines for Management of Cough , Kohno S, Ishida T, et al. The Japanese Respiratory Society guidelines for management of cough. Respirology 2006;11:S135-86. [DOI] [PubMed] [Google Scholar]
- 4.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morice AH, McGarvey L, Pavord I, et al. Recommendations for the management of cough in adults. Thorax 2006;61Suppl 1:i1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haque RA, Usmani OS, Barns PJ. Chronic idiopathic cough; a discrete clinical entity? Chest 2005,127:1710-3. [DOI] [PubMed] [Google Scholar]
- 7.McGarvey LP. Does idiopathic cough exist? Lung 2008;186Suppl 1:S78-81. [DOI] [PubMed] [Google Scholar]
- 8.Chung KF. Chronic cough as a neuropathic disorder. Lancet Respir Med 2013;1:414-22. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF. Gabapentin: a suppressant for refractory chronic cough. Lancet 2012;380:1540-1. [DOI] [PubMed] [Google Scholar]
- 10.Fujimura M, Ogawa H, Yasui M, et al. Eosinophilic tracheobronchitis and airway cough hypersensitivity in chronic non-productive cough. Clin Exp Allergy 2000;30:41. [DOI] [PubMed] [Google Scholar]
- 11.Birring SS. New concepts in the management of chronic cough. Pulm Pharmacol Ther 2011;24:334-8. [DOI] [PubMed] [Google Scholar]
- 12.Birring SS. Controversies in the evaluation and management of chronic cough. Concise clinical review. Am J Respir Crit Care Med 2011;183:708-15. [DOI] [PubMed] [Google Scholar]
- 13.Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med 1979;300:633-7. [DOI] [PubMed] [Google Scholar]
- 14.Fujimura M, Kamio Y, Hashimoto T, et al. Cough receptor sensitivity and bronchial responsiveness in patients with only chronic nonproductive cough: in view of effect of bronchodilator therapy. J Asthma 1994;31:463-72. [DOI] [PubMed] [Google Scholar]
- 15.Ohkura N, Fujimura M, Tokuda A, et al. Bronchoconstriction-triggered cough is impaired in typical asthmatics J Asthma 2010;47:51-4. [DOI] [PubMed] [Google Scholar]
- 16.Ohkura N, Fujimura M, Nakade Y, et al. Heightened cough response to bronchoconstriction in cough variant asthma. Respirology 2012;17:964. [DOI] [PubMed] [Google Scholar]
- 17.Fujimura M, Ohkura N, Abo M, et al. Exhaled nitric oxide levels in patients with atopic cough and cough variant asthma. Respirology 2008;13:359-64. [DOI] [PubMed] [Google Scholar]
- 18.Ing AJ, Ngu MC, Breslin AB. Chronic persistent cough and gastro-oesophageal reflux. Thorax 1991;46:479-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimura M, Ogawa H, Nishizawa Y, et al. Comparison of atopic cough with cough variant asthma: is atopic cough a precursor of asthma? Thorax 2003;58:14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujimura M, Abo M, Ogawa H, et al. Importance of atopic cough, cough variant asthma and sinobronchial syndrome as causes of chronic cough in the Hokuriku area of Japan. Respirology 2005;10:201-7. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg SD, Ainsworth JZ. Comparative morphology of chronic bronchitis and chronic sinusitis, with discussion of “Sinobronchial” syndrome. South Med J 1966,59:64-74. [DOI] [PubMed] [Google Scholar]
- 22.Knutsen AP, Bush RK, Demain JG, et al. Fungi and allergic lower respiratory tract diseases. J Allergy Clin Immunol 2012;129:280-91. [DOI] [PubMed] [Google Scholar]
- 23.Muscat I, Oxborrow S, Siddorn J.Allergic bronchopulmonary mycosis. Lancet 1988;1:1341. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa H, Fujimura M, Tofuku Y, et al. Eosinophilic pneumonia caused by Aspergillus niger: Is oral cleansing with amphotericin B efficatious in preventing relapse of allergic pneumonitis? J Asthma 2009;46:95-8. [DOI] [PubMed] [Google Scholar]
- 25.Selman M, Lacasse Y, Pardo A, et al. Hypersensitivity pneumonitis caused by fungi. Proc Am Thorac Soc 2010;7:229-36. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R, Gupta D.Severe asthma and fungi: current evidence. Med Mycol 2011;49Suppl 1:S150-7. [DOI] [PubMed] [Google Scholar]
- 27.Ward GW, Jr, Karlsson G, Rose G, et al. Trichophyton asthma: sensitisation of bronchi and upper airways to dermatophyte antigen. Lancet 1989;1:859-62. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka H, Niimi A, Matsumoto H, et al. Specific IgE response to trichophyton and asthma severity. Chest 2009;135:898-903. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa H, Fujimura M, Takeuchi Y, et al. Two cases of Schizophyllum asthma: Is this a new clinical entity or a precursor of ABPM? Pulm Pharmacol Ther 2011;24:559-62. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa H, Fujimura M, Takeuchi Y, et al. The influence of Schizophyllum commune on asthma severity. Lung 2011;189:485-92. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa H, Fujimura M, Amaike S, et al. Seasonal chronic cough with sputum eosinophilia caused by Trichosporon cutaneum (Trichosporon asahii). Int Arch Allergy Immunol 1998;116:162-5. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa H, Fujimura M, Matsumoto Y, et al. A case of atopic cough by Pichia guilliermondii. Nihon Kokyuki Gakkai Zasshi 1999;37:209-13. [Google Scholar]
- 33.Ogawa H, Fujimura M, Myou S, et al. Eosinophilic tracheobronchitis with cough hypersensitivity caused by Streptomyces albus antigen. Allergol Int 2000;49:83-7. [Google Scholar]
- 34.Ogawa H, Fujimura M, Tofuku Y.Isolated chronic cough with sputum eosinophilia caused by Humicola fuscoatra antigen: The importance of environmental survey for fungus as an etiologic agent. J Asthma 2002;39:331-6. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa H, Fujimura M.Fungal culture from pharyngeal swab and seasonal influence in patients with chronic non-productive cough. Nihon Kokyuki Gakkai Zasshi 2006;44:168-72. [PubMed] [Google Scholar]
- 36.Ogawa H, Fujimura M, Takeuchi Y, et al. Two cases of atopic cough successfully treated by oral cleansing with amphotericin B relation to Basidiomycetes detected from pharyngeal swab. Allergol Int 2004;53:193-6. [Google Scholar]
- 37.Ardizzoia A, Fujimura M, Tofuku Y.Treatment of atopic cough caused by Basidiomycetes antigen with low dose itraconazole. Lung 2004;182:1-8. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa H, Fujimura M, Takeuchi Y, et al. Efficacy of itraconazole in the treatment of patients with chronic cough whose sputa yield basidiomycetous fungi—Fungus-associated chronic cough (FACC). J Asthma 2009;46:407-12. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa H, Fujimura M, Takeuchi Y, et al. Sensitization to Bjerkandera adusta enhances severity of cough symptom in patients with fungus-associated chronic cough (FACC). Med Mycol J 2011;52:205-12. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa H, Fujimura M, Ohkura N, et al. Chronic cough management: dealing with a sensation of mucus in the throat. Respirology 2013;18:732. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa H, Fujimura M, Takeuchi Y, et al. Is Bjerkandera adusta important to fungus-associated chronic cough (FACC) as an allergen? Eight cases’ report. J Asthma 2009;46:849-55. [DOI] [PubMed] [Google Scholar]
- 42.Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73-9. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa H, Fujimura M, Takeuchi Y, et al. The importance of basidiomycetous fungi cultured from the sputum of chronic idiopathic cough—A study to determine the existence of recognizable clinical patterns to distinguish CIC from non-CIC. Respir Med 2009;103:1492-7. [DOI] [PubMed] [Google Scholar]
- 44.Gregory PH, Hirst JM. Possible role of Basidiospores as air-borne allergens. Nature 1952;170:414. [DOI] [PubMed] [Google Scholar]
- 45.Lopez M, Salvaggio JE, Butcher BT. Allergenicity and immunogenicity of Basidiomycetes. J Allergy Clin Immunol 1976;57:480-8. [DOI] [PubMed] [Google Scholar]
- 46.Helbling A, brander KA, Horner WE, et al. Allergy to basidiomycetes. Chem Immunol 2002,81:28-47. [DOI] [PubMed] [Google Scholar]
- 47.Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5' end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol 1997;35:1216-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Madden TL. PowerBLAST: A new network BLAST application for interactive or automated sequence analysis and annotation. Genome Res 1997;7:649-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Makino S, Kobayashi S, Miyamoto T, et al. The standardized method for antigen inhalation tests on bronchial asthma and hypersensitive pneumonitis. Arerugi 1982;31:1074-6. [PubMed] [Google Scholar]
- 50.Wang Y, Vazquez-Duhalt R, Pickard MA. Effect of growth conditions on the production of manganese peroxidase by three strains of Bjerkandera adusta. Can J Microbiol 2001;47:277-82. [DOI] [PubMed] [Google Scholar]
- 51.Kimura Y, Asada Y, Oka T, et al. Molecular analysis of a Bjerkandera adusta lignin peroxidase gene. Appl Microbiol Biotechnol 1991;35:510-4. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa H, Fujimura M, Takeuchi Y, et al. Possible roles of 2 basidiomycetous fungi in allergic fungal respiratory disease. J Allergy Clin Immunol 2012;130:279-80. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa H, Fujimura M, Makimura K, et al. Role of fungal colonization for sensitization in asthma. Clin Exp Allergy 2012;42:1540-1. [DOI] [PubMed] [Google Scholar]
- 54.Ogawa H, Fujimura M, Ohkura N, et al. Clinical experience with low-dose itraconazole in chronic idiopathic cough. Cough 2013;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sautour M, Sixt N, Dalle F, et al. Profiles and seasonal distribution of airborne fungi in indoor and outdoor environments at a French hospital. Sci Total Environ 2009;407:3766-71. [DOI] [PubMed] [Google Scholar]
- 56.Ogawa H, Fujimura, Ohkura N, et al. Is Bjerkandera allergy affected by the arrival of yellow sand dust? Allergol Int 2013;62:517-8. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa H, Fujimura M, Takeuchi Y, et al. Integrated research on the association between climate change and Bjerkandera allergy. J Allergy Clin Immunol Pract 2013;1:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization. Am J Respir Crit Care Med 2009;179:11-8. [DOI] [PubMed] [Google Scholar]
- 59.González GM, Sutton DA, Thompson E, et al. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob Agents Chemother 2001;45:633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chowdhary A, Kathuria S, Singh PK, et al. Molecular characterization and in vitro antifungal susceptibility profile of Schizophyllum commune, an emerging basidiomycete in bronchopulmonary mycoses. Antimicrob Agents Chemother 2013;57:2845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [DOI] [PubMed] [Google Scholar]
- 62.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003;58:339-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niimi A, Ogawa H. The Japanese version of Leicester Cough Questionnaire (LCQ). The 11th Japanese cough conference. Abstract (in Japanese); 2009:22. [Google Scholar]
- 64.Deary IJ, Wilson JA, Harris MB, et al. Globus pharyngis: development of a symptom assessment scale. J Psychosom Res 1995;39:203-13. [DOI] [PubMed] [Google Scholar]
- 65.Ogawa H, Fujimura M, Takeuchi Y, et al. Chronic cough management: dealing with a sensation of irritation in the throat. Respirology 2013;18:1278-9. [DOI] [PubMed] [Google Scholar]
- 66.Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice 2011;25:596-601. [DOI] [PubMed] [Google Scholar]
- 67.Chamberlain S, Garrod R, Birring SS. Cough suppression therapy: Does it work? Pulm Pharmacol Ther 2013;26:524-7. [DOI] [PubMed] [Google Scholar]
- 68.Ogawa H, Fujimura M, Takeuchi Y, et al. Dealing with cough-related laryngeal sensations for a substantial reduction in chronic cough. Pulm Pharmacol Ther 2014;27:127-8. [DOI] [PubMed] [Google Scholar]
- 69.Ogawa H, Fujimura M, Ohkura N, et al. Chronic cough in a tsunami-affected town. Pulm Pharmacol Ther 2012;25:11. [DOI] [PubMed] [Google Scholar]


