Abstract
Purpose
To investigate whether arterial spin labeling (ASL) MRI is sensitive to changes by pharmacologically induced vasodilation and vasoconstriction in rat kidneys.
Materials and Methods
Changes in renal cortical blood flow in seven rats were induced by Adenosine infusion (vasodilation) and L-NAME injection (vasoconstriction). All imaging studies were performed on a 3T scanner using a FAIR-TrueFISP sequence for the ASL implementation. The acquisition length for each ASL scan was 6 minutes. Cortical perfusion rates were calculated using regions-of-interest analysis, and the differences in perfusion rates during baseline, vasodilation, and vasoconstriction were compared and assessed for statistical significance.
Results
Compared to the baseline, an average of 94 mL/100g/min increase and 157 mL/100g/min decrease in cortical perfusion was observed following adenosine infusion and L-NAME administration respectively. The changes in cortical perfusion were significant between baseline and vasodilation (p < 0.05), baseline and vasoconstriction (p < 0.01), and vasodilation and vasoconstriction (p < 0.01).
Conclusion
ASL is sensitive to pharmacologically induced perfusion changes in rat kidneys at doses comparable to current use. The preliminary results suggest the feasibility of ASL for investigating renal blood flow in a variety of rodent models.
Keywords: Renal Perfusion, ASL, Rat Kidney, Adenosine, L-NAME, MRI
INTRODUCTION
In recent years, non-contrast functional MRI approaches (e.g. BOLD, diffusion, perfusion) applied to kidneys are becoming accepted to evaluate renal function (1), which now play a key role in translational research (2). This includes arterial spin labeling (ASL), a non-invasive MRI technique that uses water molecules in blood as an endogenous tracer to assess tissue perfusion (3). ASL has been validated in both humans (4) and animal (5) models to provide quantitative assessment of renal tissue blood flow. Recently, perfusion estimates in human with ASL were shown to demonstrate significant differences between healthy and patients with chronic kidney disease (CKD) (6). Reduced blood flow is implicated in the initiation and progression of CKD (7). Although contrast enhanced perfusion MRI is feasible to evaluate relative blood flow in patients, the risk of developing nephrogenic systemic fibrosis (8) limits its use in subjects with compromised renal function. ASL thus provides a viable alternative.
Small animal models are often used to provide better understanding of the pathophysiology of the disease and its progression (9). Compared to human and large animals, the smaller anatomical size of the kidneys in rats and/or mice makes the existing challenges in ASL such as peristaltic and respiratory motion, perfusion sensitivity, and susceptibility artifacts arising from air-tissue interface in the abdomen more severe. Despite these technical difficulties, several studies have shown the initial feasibility of ASL in small animal models. ASL was shown to detect abnormal perfusion in a rat model of acute kidney injury (10) and transplanted kidneys (11). Recently, Rajendran et al. investigated the implementation and feasibility of ASL at 7T in mice (12).
An inherent requirement of functional methods is to show acute changes following a physiological or pharmacological stimuli (13). In this study, we investigated whether ASL perfusion imaging has sufficient sensitivity to changes induced by pharmacological stimuli at commonly used doses (14).
MATERIALS AND METHODS
All animal handling and experiments were conducted under a protocol approved by the local Institutional Animal Care and Use Committee (IACUC) and in accordance with animal welfare regulations. A total of 7 Sprague Dawley rats were examined in this study.
Animal Preparation
Seven rats with weights ranging from 368 to 521 grams were obtained from Charles River (Chicago, IL) and housed at the institutional animal care facility. The animals were fed with standard rodent chow and water ad libitum. A catheter (PE-50) was placed in the femoral vein for administration of vasoactive drugs. All procedures were conducted under anesthesia using Inactin (thiobutabarbital sodium, 100 mg/kg i.p., Sigma-Aldrich, St. Louis, MO).
Pharmacological Agents
Adenosine and L-NAME (Sigma-Aldrich) were chosen in this study to induce renal blood flow changes based on prior experience with these agents (14). Adenosine is an endogenous nucleoside known to stimulate a variety of vascular receptors in the kidneys. The effect of adenosine is vasodilation when administered as intravenous infusion (15). The duration of action of adenosine is very short (16), and thus can be used repeatedly. L-NAME, a synthetic nitric oxide synthase inhibitor, was used for vasoconstriction (17,18). The duration of action for L-NAME is greater than 2 hours (19).
Perfusion Study Design
In this study, adenosine was formulated from 200 mg/kg adenosine hemisulfate salt and dissolved in 10 mL saline. An infusion pump was used to infuse the dissolved adenosine with a rate of 0.05 mL/min resulting in a dose rate of 1 mg/kg/min. The pump was situated outside the scan room and connected to the animal via silicone tubing through a waveguide. L-NAME was administered into the femoral vein as a 10 mg/kg (body weight) bolus.
Due to the rapid onset and short duration of response for adenosine (16) and the long duration of action of L-NAME (19), six perfusion measurements were made in the order illustrated in Figure 1. The Adenosine infusion was alternated between baseline (OFF) and vasodilation (ON) to test the reproducibility of the observed response. Adenosine infusion and L-NAME injection were started 10 – 15 minutes prior to the start of ASL imaging. The Adenosine infusion remained ON during the ASL acquisition.
Figure 1.
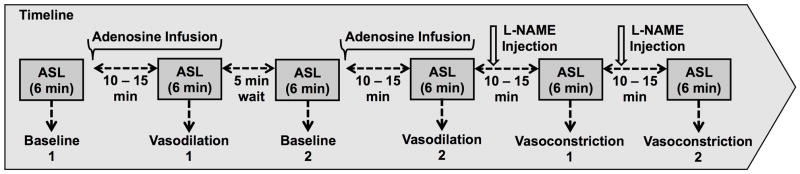
Experimental design. Baseline and vasodilation scans were repeated to assess reproducibility. The vasoconstriction scans were repeated to study the cumulative effects of L-NAME.
Imaging Protocol and Perfusion Quantification
All imaging studies were performed on a Siemens 3T scanner (MAGNETOM Verio, Siemens HealthCare, Erlangen, Germany) with an eight-channel knee coil (Siemens HealthCare) for data collection. Each animal was placed on a cushion at the center of the knee coil in a right lateral decubitus position during imaging. ASL sequence was implemented with a flow-sensitive alternating inversion recovery (FAIR) (20) preparation and a balanced steady state free precession (TrueFISP) readout (6,21). Imaging parameters were: FOV/TE/TR = 83mm/2.5ms/6s; flip angle = 60°; slice thickness = 4.5 mm; averages = 30; imaging matrix = 128 × 78 (frequency x phase); post labeling delay = 1.2s; labeling band thickness = 10mm; bandwidth = 651 Hz/Px. A selective inversion band thickness of 8mm was used to ensure proper tagging efficiency. A linear ramp catalyzation pulse was used for the TrueFISP readout. 30 control and label pairs were acquired in 6 minutes of scan time. A proton density weighted image was acquired with the same TrueFISP readout immediately following the FAIR-TrueFISP scan with a TR = 10s for perfusion quantification.
Raw data was transferred for offline post-processing using MATLAB (MathWorks, Natick, MA). Images were first aligned using FMRIB’s Linear Image Registration Tool (FLIRT, FMRIB, Oxford, United Kingdom) (22) with the first image in the perfusion series as the reference. The difference images obtained by subtracting the control from the label images were averaged into a single perfusion weighted image. Quantitative renal blood flow maps (RBF) were then calculated using a single compartment model on voxel to voxel basis
where f is the perfusion rate (in the unit of ml/100g/min), λ is the blood-tissue water partition coefficient, which is assumed to be 80 ml/100g (23), α is the inversion efficiency which is assumed to be 0.95, ΔM is the averaged difference between the control and label images and considered as perfusion weighted image, M0 is the equilibrium magnetization of the tissue (proton density). TI=1.2s is the post labeling delay time (10). T1 of 1.14 sec is assumed for the renal cortex (24).
Data Analysis
Mean perfusion rates were calculated by manual selection of regions-of-interests (ROI) on RBF maps. ROIs were defined in the renal cortex for both kidneys. Renal arteries were carefully excluded in the ROIs. Only one kidney was imaged in three rats due to the different lateral position of the kidneys. A total of 11 kidneys were used in the final analysis.
The effects of Adenosine and L-NAME were analyzed using paired t-tests. A p-value of 0.05 is considered to indicate significance. The reproducibility of the baseline and Adenosine scans were assessed using coefficient of variations (CV).
RESULTS
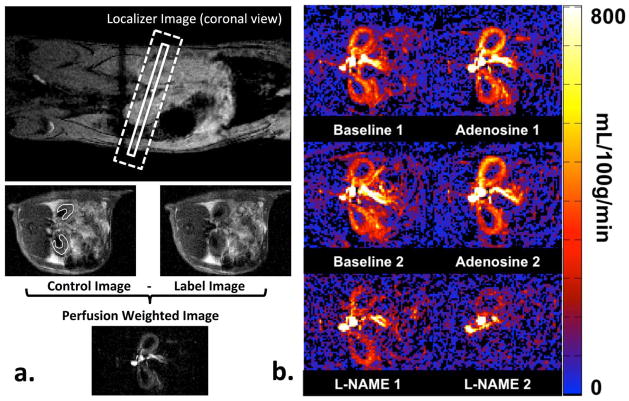
An illustration of the representative RBF map for each condition is shown in Figure 2. Renal cortex can be clearly distinguished from the renal medulla in the baseline and vasodilation scans. Retrospective motion correction and signal averaging were sufficient to minimize motion artifacts and the final RBF maps were of sufficient quality for analysis.
Figure 2.
a) Illustration of ASL MRI. The imaging slice and the FAIR selective inversion plane are marked by the solid and the dotted box on the localizer image, respectively. A pair of control and label images is shown below the localizer image. The cortical ROI used for quantitative analysis is shown on the control image. The corresponding perfusion weighted image is shown at the bottom. b) Representative renal blood flow maps of rat kidneys at each experimental condition. Note the increased cortical perfusion with adenosine infusion and the decreased cortical perfusion with L-NAME injection.
One rat died during data acquisition after the first L-NAME injection. The data analysis on the effects of L-NAME was hence based on only 6 animals. The mean and the standard deviation of the cortical perfusion rates were 363 ± 57 ml/100g/min for the first baseline scan, 427 ± 60 ml/100g/min for the first Adenosine scan, 357 ± 56 ml/100g/min for the second baseline scan, 445 ± 65 ml/100g/min for the second Adenosine scan, 218 ± 51 ml/100g/min for the first L-NAME scan, and 176 ± 48 ml/100g/min for the second L-NAME scan (Figure 3). The baseline perfusion rates for the cortical tissue were similar to reported values in previously reported studies (10,12). Vasodilation with Adenosine resulted in an average of 94 ml/100g/min increase in perfusion (26% increase) compared to baseline; and vasoconstriction with L-NAME lowered the cortical perfusion by an average of 145 ml/100g/min after the first injection of L-NAME (36% decrease) compared to baseline. The cortical perfusion further dropped an average of 40 ml/100g/min following the second L-NAME injection (46% decrease compared to the baseline). The perfusion rate drop between the first and second L-NAME injection (p < 0.05) is consistent with the long action times. The changes in perfusion rate were significant between baseline and vasodilation (p < 0.05), baseline and vasoconstriction (p < 0.01), and vasodilation and vasoconstriction (p < 0.01). The coefficients of variations were 4% and 11.6% for baseline and adenosine measurements, respectively, indicating good reproducibility.
Figure 3.
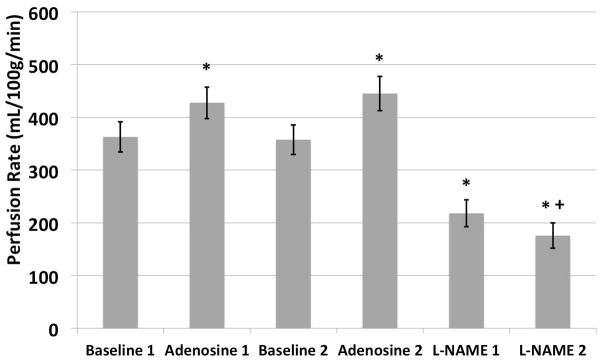
Comparisons of quantitative perfusion rates. Note the return to baseline perfusion values when the infusion of Adenosine was stopped. The increase in perfusion is comparable after each dose, and is statistically significant compared to the baseline. On the other hand, administration of L-NAME results in significant reduction in perfusion compared to baseline. Following the second administration, there is further significant reduction in perfusion. No significance difference was observed between the two baseline, as well as Adenosine scans.
* indicates significant difference (p < 0.05) comparing to the corresponding baseline scans.
+ indicates significant difference (p < 0.05) comparing to the first L-NAME scan
DISCUSSION
In healthy kidneys auto-regulation will maintain RBF and glomerular filtration rate (GFR) values over a wide range of arterial pressures. GFR varies linearly over the normal range of RBF values. In diseases, previous studies (25) have shown that RBF was found to be a strong independent predictor of the GFR. In this study, feasibility of FAIR ASL to obtain quantitative renal perfusion measurement on a clinical 3T scanner in a rodent model was demonstrated. Furthermore, we have shown that FAIR-ASL was sensitive to variations in renal perfusion caused by pharmacologically induced vasodilation and vasoconstriction. The findings suggest that it is feasible to use ASL to investigate various effects on renal blood flow in rodent models, such as contrast-induced acute kidney injury (26).
On average, the cortical perfusion increased by 26% during Adenosine infusion (at a dose rate of 1 mg/kg/min) and decreased by 36% after the first L-NAME injection (10 mg/kg bolus). In two animals, one of the two vasodilation scans during Adenosine infusion resulted in little or negative change in cortical perfusions (0.8% and −6% respectively). We do not know the exact cause for these observations, and can only speculate that it may be a consequence of pump malfunction, or a delayed response to the Adenosine. In a previous study, Granstam et. al (27) reported a 60% decrease in blood flow at similar dose using microsphere analysis following the L-NAME injection (dose), which was a larger change than we have observed. However, they have followed the bolus L-NAME injection with an infusion for 10 minutes at a rate of 10 mg/kg. In our study, the cortical perfusion decreased further by an average of 20% after the second L-NAME injection. This cumulative response was more consistent with the changes reported by Granstam et al.
The dosage of the vasoactive drugs in this study was chosen based on a previously published animal study looking for blood volume changes by contrast enhanced MRI (14). During our initial testing, we observed little (less than 3%) cortical perfusion change (data not shown) with an adenosine dose rate of 0.5 mg/kg/min (14). Subsequently, we doubled the dosage of Adenosine to 1.0 mg/kg/min. As shown, the average increase in perfusion was 94 mL/100g/min during adenosine infusion and was comparable to the averaged standard deviation in the baseline perfusion measurements of 107 mL/100g/min (this excludes the two previously mentioned instances where adenosine infusion resulted in anomalously low level of change). The need for higher dose of adenosine is consistent with the known limitation in terms of sensitivity of ASL compared to contrast enhanced studies such as the previous report (13). The response to L-NAME was more robust at the doses currently used (14).
While respiration is generally considered as a challenge for abdominal imaging, we observed very little image quality degradation. The post-processing co-registration technique was sufficient to minimize registration errors and no apparent subtraction artifacts were observed in the final perfusion maps. A thick labeling band (nearly twice the thickness as the imaging slice) was used to ensure the inversion efficiency (6). This can result in a sensitivity to transit time delay (28) and hence contribute to quantification errors. The use of adiabatic frequency offset corrected (FOCI) pulse (29) for selective inversion could reduce this effect (21). However, imaging slice profile also needs to be optimized to achieve maximum reduction in transit time effects (28). Additional saturation pulses (30) can further eliminate transit time related quantification errors.
Medullary perfusion was not analyzed in this study due to the concern of partial volume effects and the poor sensitivity of FAIR ASL to the slow flow in the medulla. This was a limitation of the ASL implementation and the use of standard clinical scanner. Alternatively, the use of higher field strength magnets dedicated for small animal imaging may allow for measuring medullary perfusion facilitated by the elongated T1 of the blood and hence allowing for longer inflow time. Alternately, higher field strengths could facilitate increased spatial resolution. However, ASL may be more sensitive to motion errors that retrospective correction techniques may not provide satisfactory results and additional techniques such as respiratory triggering (12), navigators (6,12,31), and selective averaging (32,33) may become necessary.
CONCLUSION
Data provided in this preliminary study support the feasibility of FAIR True-FISP based renal perfusion MRI in rat kidneys and demonstrate adequate sensitivity to pharmacological induced blood flow changes. This suggests feasibility of using the measurement in concert with techniques such as BOLD MRI in various models of ischemic injury (10,11,26). Further studies are necessary to demonstrate similar sensitivity in human measurements of renal perfusion by ASL.
Acknowledgments
Grant Support: NIH R01-DK053221
This study is support in part by (Blinded for review purpose).
References
- 1.Zhang JL, Morrell G, Rusinek H, et al. New magnetic resonance imaging methods in nephrology. Kidney Int. 2013 doi: 10.1038/ki.2013.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006;290(5):F958–974. doi: 10.1152/ajprenal.00114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19(7):701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ritt M, Janka R, Schneider MP, et al. Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant. 2010;25(4):1126–1133. doi: 10.1093/ndt/gfp639. [DOI] [PubMed] [Google Scholar]
- 5.Artz NS, Wentland AL, Sadowski EA, et al. Comparing kidney perfusion using noncontrast arterial spin labeling MRI and microsphere methods in an interventional swine model. Invest Radiol. 2011;46(2):124–131. doi: 10.1097/RLI.0b013e3181f5e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan H, Koktzoglou I, Prasad PV. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn Reson Med. 2013 doi: 10.1002/mrm.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–78. [PubMed] [Google Scholar]
- 8.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17(9):2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 9.D’Agati V, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nature reviews Nephrology. 2010;6(6):352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 10.Zimmer F, Zollner FG, Hoeger S, et al. Quantitative renal perfusion measurements in a rat model of acute kidney injury at 3T: testing inter- and intramethodical significance of ASL and DCE-MRI. Plos One. 2013;8(1):e53849. doi: 10.1371/journal.pone.0053849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JJ, Hendrich KS, Jackson EK, Ildstad ST, Williams DS, Ho C. Perfusion quantitation in transplanted rat kidney by MRI with arterial spin labeling. Kidney Int. 1998;53(6):1783–1791. doi: 10.1046/j.1523-1755.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajendran R, Lew SK, Yong CX, Tan J, Wang DJ, Chuang KH. Quantitative mouse renal perfusion using arterial spin labeling. NMR Biomed. 2013;26(10):1225–1232. doi: 10.1002/nbm.2939. [DOI] [PubMed] [Google Scholar]
- 13.Ji L, Li LP, Schnitzer T, Du H, Prasad PV. Intra-renal oxygenation in rat kidneys during water loading: effects of cyclooxygenase (COX) inhibition and nitric oxide (NO) donation. J Magn Reson Imaging. 2010;32(2):383–387. doi: 10.1002/jmri.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Storey P, Ji L, Li LP, Prasad PV. Sensitivity of USPIO-enhanced R2 imaging to dynamic blood volume changes in the rat kidney. J Magn Reson Imaging. 2011;33(5):1091–1099. doi: 10.1002/jmri.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther. 2005;315(3):1150–1157. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- 16.Biaggioni I. Clinical and molecular pharmacologic characteristics of adenosine-induced vasodilation. Clinical pharmacology and therapeutics. 2004;75(3):137–139. doi: 10.1016/j.clpt.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Storey P, Kim D, Li W, Prasad P. Kidneys in hypertensive rats show reduced response to nitric oxide synthase inhibition as evaluated by BOLD MRI. J Magn Reson Imaging. 2003;17(6):671–675. doi: 10.1002/jmri.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li LP, Ji L, Santos E, Dunkle E, Pierchala L, Prasad P. Effect of nitric oxide synthase inhibition on intrarenal oxygenation as evaluated by blood oxygenation level-dependent magnetic resonance imaging. Invest Radiol. 2009;44(2):67–73. doi: 10.1097/RLI.0b013e3181900975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YX, Poon CI, Pang CC. Vascular pharmacodynamics of NG-nitro-L-arginine methyl ester in vitro and in vivo. J Pharmacol Exp Ther. 1993;267(3):1091–1099. [PubMed] [Google Scholar]
- 20.Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34(3):293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 21.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51(2):353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 23.Karger N, Biederer J, Lusse S, et al. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn Reson Imaging. 2000;18(6):641–647. doi: 10.1016/s0730-725x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 24.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3. 0 T: preliminary results. Radiology. 2004;230(3):652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 25.Torres VE, King BF, Chapman AB, et al. Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(1):112–120. doi: 10.2215/CJN.00910306. [DOI] [PubMed] [Google Scholar]
- 26.Li LP, Franklin T, Du H, et al. Intrarenal oxygenation by blood oxygenation level-dependent MRI in contrast nephropathy model: effect of the viscosity and dose. J Magn Reson Imaging. 2012;36(5):1162–1167. doi: 10.1002/jmri.23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granstam SO, Granstam E. Endothelin-induced changes in blood flow in STZ-diabetic and non-diabetic rats: relation to nitric oxide synthase and cyclooxygenase inhibition. The journal of physiological sciences : JPS. 2011;61(6):497–505. doi: 10.1007/s12576-011-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pell GS, Lewis DP, Ordidge RJ, Branch CA. TurboFLASH FAIR imaging with optimized inversion and imaging profiles. Magn Reson Med. 2004;51(1):46–54. doi: 10.1002/mrm.10674. [DOI] [PubMed] [Google Scholar]
- 29.Ordidge RJ, Wylezinska M, Hugg JW, Butterworth E, Franconi F. Frequency offset corrected inversion (FOCI) pulses for use in localized spectroscopy. Magn Reson Med. 1996;36(4):562–566. doi: 10.1002/mrm.1910360410. [DOI] [PubMed] [Google Scholar]
- 30.Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 31.Song R, Loeffler RB, Hillenbrand CM. Improved renal perfusion measurement with a dual navigator-gated Q2TIPS fair technique. Magn Reson Med. 2010;64(5):1352–1359. doi: 10.1002/mrm.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan H, Maldjian JA, Pollock JM, et al. A fast, effective filtering method for improving clinical pulsed arterial spin labeling MRI. J Magn Reson Imaging. 2009;29(5):1134–1139. doi: 10.1002/jmri.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gardener AG, Francis ST. Multislice perfusion of the kidneys using parallel imaging: image acquisition and analysis strategies. Magn Reson Med. 2010;63(6):1627–1636. doi: 10.1002/mrm.22387. [DOI] [PubMed] [Google Scholar]