Figure 1.
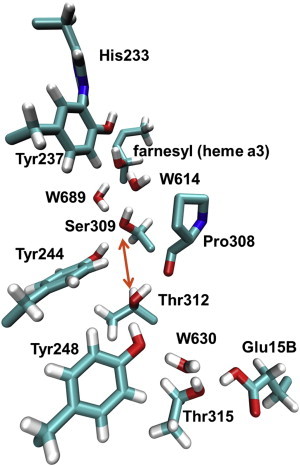
Proton wire in the K-channel analog in CcO of T. thermophilus. The channel runs from Glu-15B to Tyr-237, with one gap of 4.9 Å in the H-bond chain between Thr-312 and Ser-309 (indicated by an orange arrow). For proton transport, a proton hole is presumably transported from Tyr-237 to Glu-15B via farnesyl, W614, Ser-309, Tyr-244, Thr-312, Tyr-248, W630, and Thr-315. Coordinates were taken from crystal structure PDB 3S8F (6) and hydrogen atoms were added with CHARMM (19). In addition to the channel residues, Pro-308 is depicted (without hydrogens) because its backbone CO group accepts an H-bond from Thr-312, and His-233 is depicted because it is covalently bound to Tyr-237. For clarity, the farnesyl chain of heme a3 is shown only partially. To see this figure in color, go online.
