Figure 1.
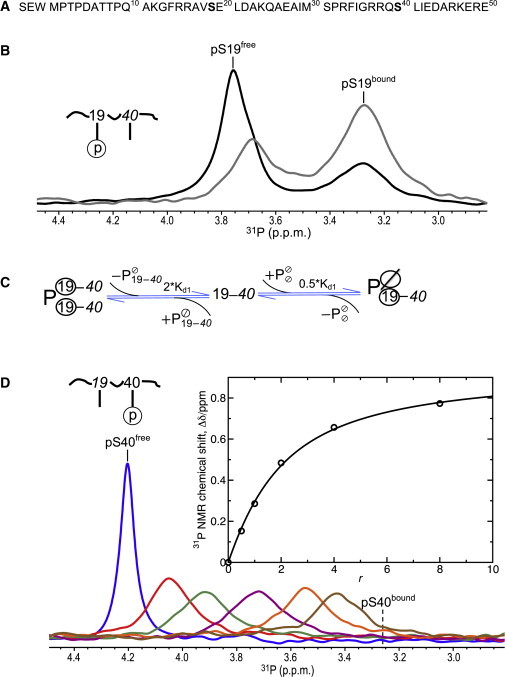
Binding of singly phosphorylated hTH1–50 peptides to 14-3-3ζ. (A) Amino-acid sequence of the hTH1–50 peptide with S19 and S40 highlighted (boldface). (B) 31P spectra of pS19-hTH1–50 at 0.83 mM peptide concentration and molar ratios of 14-3-3ζ to peptide (r) 0.5:1 (black) and 1:1 (gray). (C) Schematic depiction of pS19-hTH1–50 (denoted by 19–40) binding to the 14-3-3ζ homodimer (free protein dimer is depicted by , where Ø indicates an empty phosphoserine binding site). (Circled) Bound pS19 residues. (D) 31P spectra of pS40-hTH1–50 at 0.71 mM peptide concentration and molar ratios (r) of 14-3-3ζ to peptide of 0:1 (blue), 0.5:1 (red), 1:1 (dark green), 2:1 (purple), 4:1 (orange), and 8:1 (brown). (Inset) Chemical shift changes upon 14-3-3ζ binding (Δδ) are plotted as a function of molar ratio (r). The curve obtained by fitting Δδ to the quadratic equation shown in Eq. 2 is shown. To see this figure in color, go online.
