Figure 2.
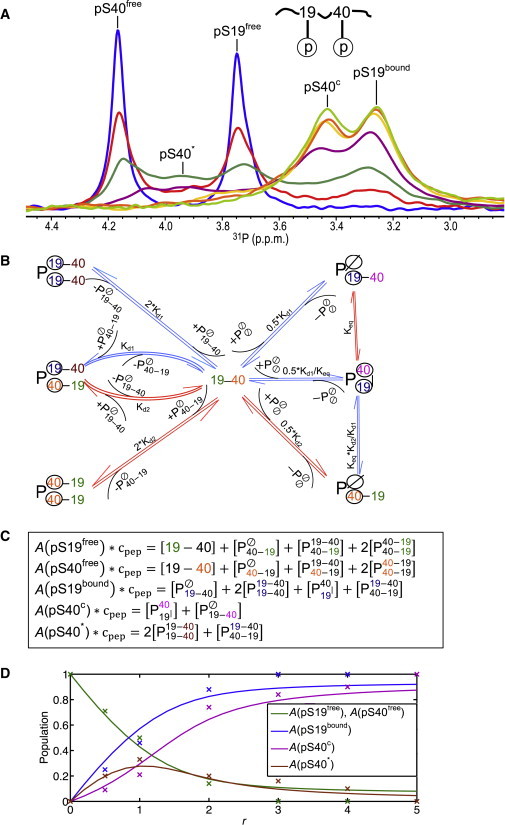
Binding of doubly phosphorylated peptide to 14-3-3ζ. (A) 31P spectra of pS19pS40-hTH1–50 at 0.83 mM and the molar ratio of 14-3-3ζ to the peptide (r) was 0:1 (blue), 0.5:1 (red), 1:1 (dark green), 2:1 (purple), 3:1 (yellow), 4:1 (orange), or 5:1 (light green). (B) Binding scheme of pS19pS40-hTH1–50 (denoted by 19–40) with respect to the 14-3-3ζ homodimer (). Dissociation constants Kd1 and Kd2 correspond to the values determined for the singly phosphorylated peptides. (Blue arrows) Equilibria for which slow exchange on the 31P chemical shift scale is observed; (red arrows) equilibria for which fast exchange on the 31P chemical shift scale is observed. (Circled) Bound phosphorous groups. Phosphorous groups contributing intensity to the same peak in the NMR spectra are shown in the same color. (C) Equations governing the relative peak intensities (denoted as “A”), based on the scheme depicted in panel B; each phosphopeptide contributes to one or more of the five peaks. The specific phosphoserine that contributes to intensity of a particular peak is colored in the same color as in panel B. (D) Populations of phosphoserine species that contribute to the experimentally observed peak intensities (x) for different 14-3-3ζ to peptide molar ratios, r. (Continuous lines) For comparison, the theoretically predicted populations, based on the binding model in panel B using Kd1 = 0.15 mM, Kd2 = 1.0 mM, and Keq = 1.5, are plotted. To see this figure in color, go online.
