Figure 2.
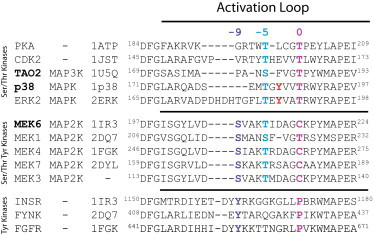
Sequence alignment of activation loop sequences of MAP kinases. The sequences of activation loops of protein kinases can be aligned with respect to conserved sequences at either end and the DFG box and the APE sequence. Zero is defined as the position of the substrate binding residue—a threonine in Ser/Thr kinases. The tyrosines (red) in p38 MAPK and ERK MAPK do not align with any of the phosphorylation sites in either Ser/Thr kinases or Tyr kinases. The threonine (pink) of p38 and ERK2 aligns with the primary activating phosphorylation in other Ser/Thr kinases. The two phosphorylation sites on MAP2Ks align with the primary activating phosphorylation sites in both Ser/Thr (blue) and Tyr (purple) kinases. (Boldface) Kinases in the p38 module. The Protein Data Bank (PDB) files are indicated. To see this figure in color, go online.
