Figure 5.
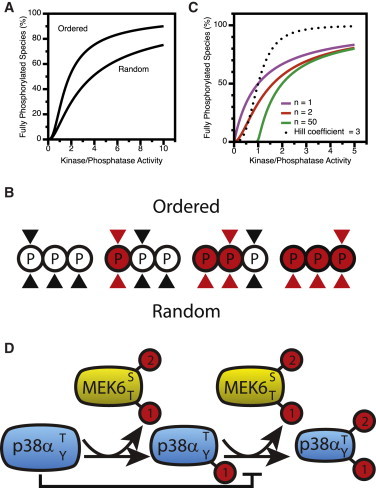
Multisite phosphorylation. (A) The sequence of phosphorylation and dephosphorylation events impacts the steady-state concentration of fully phosphorylated product based on Eqs. 6 and 9 from Salazar and Höfer (45). (B) An example of random versus ordered phosphorylation in a substrate with three phosphorylation sites. (Open circles) Unphosphorylated sites; (red circles) phosphorylated. (Black triangles) Potential placement of the phosphorylation site in the kinase active site; (red triangles) indicate potential placement of the phosphorylation site in the phosphatase active site. Ordered reactions exhibit a stronger switchlike steady-state response to stimulation because increasing overall phosphorylation does not decrease available sites or increase phosphatase sites as quickly. (C) The impact of multiple phosphorylation sites on the percentage of fully phosphorylated substrate as a function of kinase/phosphatase ratio as based on Eq. 9 in Gunawardena (68). The Hill function is shown for comparison: % fully phosphorylated kinase = (K/P)3/1 + (K/P)3, where K is the activity of the kinase, and P the activity of the phosphatase. Higher numbers of phosphorylation sites do not increase sigmoidicity. (D) Positive feedback is inherent to each MAP kinase tier by merit of its architecture. For each tier of the cascade, the rate of the second reaction is inhibited by the amount of unphosphorylated substrate. As the reaction progresses, the amount of unphosphorylated substrate decreases, relieving inhibition and driving positive feedback. To see this figure in color, go online.
