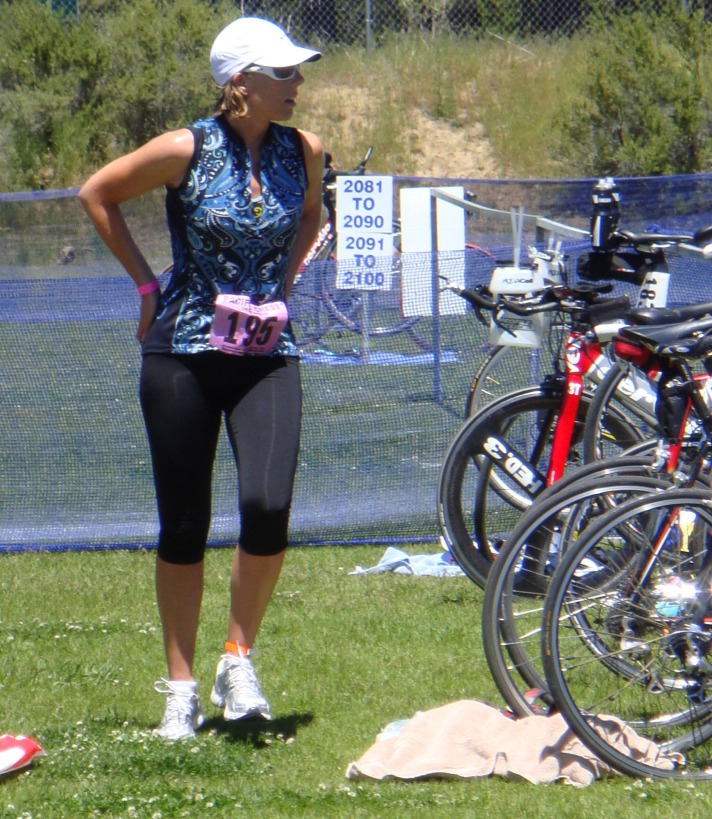Abstract
Total Hip Athroplasty (THA) is a common procedure in orthopedic surgery to address severe osteoarthritis (OA) in the hip joint. With the burgeoning “baby boomer” generation and older athletes who wish to return to competitive levels of sports, understanding how sporting activity affects THA outcomes is becoming exceptionally important. The purpose of this review is to characterize the current recommendations and risks for returning to sports after THA, as well as discuss the implications of the changing demographic and level of expectation on rehabilitation paradigms.
Although the actual risks associated with participating in sports after THA are unknown, there are concerns that higher levels of physical activity after THA may increase risk for fracture, dislocation and poor long‐term outcomes. Evidence surrounding the specific effect of sporting activity on wear after THA is conflicting. Newer alternatives such as metal‐on‐metal hip resurfacing are expected to provide better durability but there are concerns of systemic metal ions from mechanical wear, although the impact of these ions on patient health is not clear.
Tracking outcomes in patients participating in higher level activities after THA presents a problem. Recently the High Activity Arthroplasty Score has been developed in response to the need to quantify higher level of physical activity and sports participation after joint arthroplasty. This measure has been shown to have a higher ceiling effect than other common outcome measures.
There is little prospective evidence regarding the likelihood of poor clinical outcomes with higher level of sporting activity. There is some evidence to suggest that wear may be related to activity level, but the impact on clinical outcomes is conflicting. When advising an athlete considering returning to sport after THA, consider their preoperative activity level, current physical fitness, and specific history including bone quality, surgical approach and type of prosthesis.
Level of evidence:
5
Keywords: Activity, arthroplasty, high‐impact, joint replacement, sports
INTRODUCTION
Total Hip Athroplasty (THA) is a common procedure in orthopedic surgery used to address severe osteoarthritis (OA) in the hip joint. Originally considered a salvage procedure, the initial goal was simply to allow people to perform basic activities of daily living without experiencing excruciating pain. The first several decades of surgical experimentation provided unsatisfactory outcomes and an alarming number of prosthetic failures.1 As surgical techniques and biomaterials have improved in the past 30 years, THA is now the standard procedure to manage the pain of end‐stage hip osteoarthritis and the majority of patients report greater quality of life after the procedure.2
The incidence and utilization of THA has dramatically grown in the past 10 years. This is due in part to enhanced surgical competency with the procedure and an increase in the number of patients who are candidates for THA. Recent estimates found that nearly one in four individuals will develop symptomatic hip OA in their lifetime.3 In 2010, there were an estimated 301,000 primary THA procedures and an additional 49,860 revision THA surgeries.4 The trajectory for THA continues to increase and it is estimated that the annual incidence of primary and revision THA will exceed 575,000 by the year 2020.4 Although there has been, and will continue to be a dramatic increase in the number of THAs performed each year, there is a concurrent substantial shift in the patient demographic. The population of patients undergoing the procedure is becoming increasingly younger.5 From 2001 to 2007 in the United States, the incidence rate of THA in patients between the ages of 50 and 59 increased by 50%.5 This far outpaces the incidence in persons 60‐69 (14.9%) and 70‐79 (8.6%). As a greater number of younger patients undergo THA, the expectations for to return to high‐level activities, including sports and physically demanding vocations, will continue to increase. (Figure 1)
Figure 1.
37 year‐old female triathlete, after THA.
THA is most commonly performed for degenerative articular disease in older adults; however, the number of patients younger than 60 is steadily increasing. THA is often performed in these younger patients for a variety of additional precipitating pathologies, including post‐traumatic OA, rheumatoid arthritis, hip dysplasia, and avascular necrosis. With the burgeoning “baby boomer” generation and older athletes who wish to return to competitive levels of sports, understanding how sporting activity affects THA outcomes is becoming exceptionally important. Conversely, understanding how undergoing THA may reduce or increase an individual's likelihood of continuing with sports is also of interest to clinicians, surgeons and most importantly, to patients. The success of THA has prompted commercial and marketing campaigns targeted at younger individuals who plan to return to higher level of activity after surgery, even though long‐term outcome data are lacking in this population. There has been considerable press about athletes who return to sports after this invasive procedure and the list includes professional ballet dancers, world‐ranked tennis players, and masters‐level golfers.6 Some of these professional athletes have been able to return at levels of function that met or exceeded pre‐operative ability,7,8 while other athletes have been significantly less successful.9 The purpose of this clinical commentary is to summarize the current recommendations and risks for returning to sports after THA, as well as discuss the implications of the changing demographic and level of expectation on rehabilitation paradigms.
CURRENT RECOMMENDATIONS FOR SPORT AFTER THA
There is a lack of empirical data to support the type of activities that are safe and feasible for patients after THA. The current recommendations of allowable or recommended activities are derived from surveys of hip and knee surgeons based on clinical experience and preference, not prospective and retrospective analyses. In 2005, data from 614 surgeons were collected to determine activities that were “allowed,” “allowed with experience,” or “not allowed”.10 These responses were variable, but in general, low‐impact activities such as swimming, bowling, stationary biking, dancing, rowing and walking were allowed. Downhill and cross‐country skiing, weightlifting, ice‐skating and pilates were activities that were allowed with experience. There was a general consensus that racquetball/squash, jogging, contact sports, high‐impact aerobics, baseball/softball and snowboarding were not allowed. For the allowable activities, most surgeons recommended that patients could return to these activities 3 to 6 months after surgery, although approximately 1/3 of surgeons recommended 1 to 3 months after surgery as an acceptable timeframe. Participation in low‐impact sports such as walking, golf and bowling have been shown to be safe and not impair short‐term outcomes.11
A recent study had 139 surgeons categorize recommendations for the allowed frequency of similar activities. The allowable frequencies were classified as either “unlimited,” “occasional (1‐2 times per month),” or “discouraged”.12 The results were similar to previous work and low‐impact activities were generally recommended in unlimited amounts. These activities included swimming, walking, golf, doubles tennis, and cycling.(Figure 2) The majority of respondents recommended that jogging, sprinting and skiing difficult terrain were discouraged. Among all the respondents, none indicated that there was scientific evidence for his or her recommendations.
Figure 2.
37 year‐old female triathlete, participating in cycling, after THA.
In 2012, Delasotta et al.13 evaluated whether patients who underwent THA adhered to the current recommended activities from the 2005 survey.10 Of the 62 patients surveyed, the majority of patients reported that they participated in recommended activities; while only two patients said that they participated in activities that were discouraged by their surgeon (jogging and squash). When asked why higher impact activities were not resumed, the main reasons were fear (28.6%) and physician recommendation (25.7%). Pain, fatigue, and lack of interest were not the primary reasons for stopping higher‐level activities. The impact of fear on resumption of activity was also reported by Abe et al.14 These authors found that in patients who underwent hip resurfacing and wanted to return to jogging, 61% did not do so because of anxiety, while only 15% reported that pain kept them from returning. The hip resurfacing is a more conservative surgical procedure in which the acetabulum and a portion of the femoral head are replaced, while preserving bone stock in the femoral neck and shaft that is usually sacrificed in a traditional THA. In an analysis of 285 patients who underwent THA, Huch et al15 found that 56% of patients stopped participating in sports “as a precaution, to go easy on the artificial joint.” These studies highlight that fear of movement and patient education are important considerations in post‐operative rehabilitation. Factors other than endurance and pain need to be considered when evaluating patients who wish to return to sport.
ACTIVITY‐RELATED RISK AFTER THA
Although the actual risks associated with participating in sports after THA are unknown, there are concerns high‐impact or frequent physical activity after THA may increase risk for fracture, dislocation, and thereby create poor long‐term outcomes. It is difficult to quantify these risks in patients after THA for several reasons. Factors other than level of activity, such as bone quality or insufficient rehabilitation progression may influence these injuries, long‐term outcome data in young patients is not available for contemporary prostheses and biomaterials, and outcomes cannot be ethically tested in randomized controlled studies. Therefore, providers must rely on retrospective and epidemiological evaluations of outcomes after THA.
Dislocation
One of the primary concerns of patients and surgeons is dislocation after THA. Because the hip joint undergoes large excursions of movement in all three planes, the incidence of dislocation is higher in THA than in other arthroplasty procedures. Incidence of dislocation typically ranges from 3 to 5% after all THA procedures.16,17 although this number varies depending on the characteristics of the patient sample. Posterior approaches have been shown to increase the likelihood of early and late‐stage dislocation, although soft tissue repair of the joint capsule and rotator muscles reduce this risk.18 The size of the femoral head has also been a factor in dislocation risk, with greater diameter femoral head size associated with reduced risk of dislocation.19 It is estimated 10.4% to 22.5% of all THA revisions are due to hip dislocation20,21, making it one of the most common causes for THA revision. The risk of dislocation is greatest in the first 10 weeks after THA and increases dramatically in patients with a previous history of dislocation.22 Despite the prevalence of this risk, to date there is little evidence supporting or refuting a connection between dislocation risk and the intensity or frequency of post‐operative activity. Results from studies describing the causes of hip dislocation have shown that low‐impact activities such as getting into or out of bed, rising or sitting on the toilet, and putting on shoes or socks are associated with dislocation, not higher level sporting activities.23 Ollivier et al found no statistical difference in dislocation rates in subjects who reported participating in higher impact activities after THA when compared to a group with less reported physical activity (dislocation rate 1.4% in high‐impact group vs. 2.14% in the low activity group, p=0.50).24
Fractures
Periprosthetic fractures are a concern after THA as they lead to greater mortality.25 Fractures may be a particular risk during sporting activities because large torques applied to the femoral head during running, cutting and jumping activities are transmitted to the stem located within the shaft of the femur. However, little documented evidence suggests that patients who return to sports after THA are at greater fracture risk. Two published cases of periprosthetic fracture of the femur report occurrence during winter activities.26 One case involved a skiing accident that occurred in difficult terrain while the other case described a head‐on collision of two snowmobiles. In either case it could be argued that the fracture had little if any relation to the history of THA and both patients made a complete recovery.
Aseptic Loosening
Aseptic loosening is the primary cause of revision in THA.27,28 This occurs when the bone surrounding the prosthesis degrades in the absence of a known infection. It most commonly results from periprosthetic osteolysis in which the surrounding bone is removed as part of the normal homeostasis process, but it is not subsequently replaced. This is process is often the direct result of accumulated particulate debris from polymethyl methacrylate (bone cement) and polyethylene (plastic spacer), but may also arise as a result of increased forces transmitted through the stem of the prosthesis. As with other concerns, little has been published on the true risk of aseptic loosening of the implant with higher impact activities.
Lefevre et al29 found that 29 of 38 patients (mean age at the time of surgery was 63 ± 7.2 years) returned to judo practice following THA, although none returned to competitive judo. In this sample, two patients required revision for aseptic loosening 6 and 9 years after THA. Two older studies evaluated and reported on the relationship between activity and long‐term outcomes after THA. Dubs et al30 found that patients who returned to higher levels of sporting activity actually had a lower incidence of revision due to aseptic loosening. Cornell et al31 followed 85 consecutive generally active patients under the age of 55 (a total of 101 hips) for a minimum of 10 years. At the 10‐year follow up, only two patients required revision for acetabular implant loosening. It should also be noted that these were cemented prostheses implanted during the 1970s. It would be reasonable to expect a higher THA survival rate today with more modern surgical techniques and materials.
A more recent study evaluated revision in a sample of patients who required THA revision within a 10 year follow‐up period. A greater likelihood of early revision for aseptic loosening was found in the younger sample.32 Despite this trend in age, the subjects in this study had a variety of conditions that necessitated the index THA, including OA, rheumatoid arthritis, osteonecrosis, dysplasia and post‐traumatic OA. Subjects with avascular necrosis and dysplasia were younger than those with OA and were also more likely to require revision. Caution should be used when making conclusions about activity level and implant failures from younger versus older patients because age is not necessarily correlated with activity level after THA.33 No studies have looked specifically at implant loosening with higher impact and sporting activities.
Polyethylene Wear
One of the main concerns of resuming regular high‐impact physical activity after conventional total joint arthroplasty is mechanical degradation of the polyethylene spacers that are used in most traditional joint arthroplasties. These materials are built to withstand large cyclical forces, but the wear rate of this plastic is related to the amount of use, which has been established by in vitro34 and in vivo studies.35 As the polyethylene wears, it creates particulate debris that can remain localized within the joint or spread to adjacent tissue. This can lead to sensations of pain and instability,36 osteolysis and subsequent aspetic loosening,37, and regional granulomas and cysts.38 Polyethylene wear and the associated prosthetic loosening are the most common causes of post‐operative failure and the need for revision surgery. Therefore, activities that potentially expedite the wear through increased frequency or magnitude of loading (such as high impact sports) is a primary concern. To date there is limited information on the specific relationship between wear and sporting activities.
One analysis of younger individuals (mean 33.9 years old) after THA found that the 25‐year survival rate for THA prostheses was 74% and 59% for the femoral and acetabular components, respectively.39 The risk of revision was related to the amount of wear of the acetabular component, which averaged 0.12 mm/yr. However, these procedures were performed between 1966 and 1978 before the advent of newer iterations of ultrahigh molecular weight polyethylene liners that are more resistant to wear.40 A more recent long‐term analysis of THA patients under 50 years of age found that the prosthesis survival after a mean of 28.4 years was 90% and 66% for femoral and acetabular components, respectively.41 The mean Harris hip score in this sample was 89 out of 100, although 25% of patients reported thigh pain. Polyethylene wear rate was found to be 0.18 mm/yr in the sample. It is important to note that neither of these authors accounted for activity or sport participation, but only evaluated a younger patient sample.
The clinical evidence supporting the relationship between activity level and wear rates are mixed. Schmalzried et al35 assessed polyethylene wear in 37 hip replacements via digital images while accounting for activity using a pedometer. Activity (number of steps) was related to wear with a 90% confidence level. Contrary to this finding, Sechriest et al33 found no relationship between walking activity (number of steps) and wear rate of the prosthesis in younger or older adults after THA. These authors found that there was not a significant difference in activity post‐operative activity level between the older and younger subjects, suggesting that outcomes and wear rates evaluated on age alone as a basis for greater activity may be misleading. Although Schmalzried did describe a significant relationship between age and activity after THA in a previous study (p=0.048), there was little perceivable relationship between the variables and the authors acknowledged that there was substantial variability in the scatterplot.42 The authors did not report the correlation coefficient (r‐value) for the relationship between age and steps per day. While both of these studies evaluated the amount of activity, they did not quantify the magnitude or intensity of the activities. It is possible that greater impact of any duration is the key component to prosthetic wear.
Evidence surrounding the specific effect of high‐impact sporting activity on wear after THA is also conflicting. Biomechanical studies evaluating joint contact forces have found that downhill skiing produces internal forces that are up to 7.8 times greater than body weight.43 Given the high forces during this activity, it is conceivable that this sport would generate a considerable amount of prosthetic wear. Gschwent et al44 conducted a matched‐cohort study of 100 subjects in which one group routinely participated in skiing, while the other group did not. The authors found that 10 years after surgery, there was a greater rate of polyethylene wear in the skiing group, particularly among the most active patients. However, five years after THA there was a greater likelihood of osteolysis and loosening in those who did not ski and the number of complications was greater in the less active cohort. The authors concluded that although high levels of physical activity may contribute to wear, it did not affect the incidence of loosening. Ollivier et al conducted a retrospective study of 210 patients who participated in high‐impact sports (n=70) or low‐level activity (n=140).24 These groups were defined based on the subject's University of California Los Angeles (UCLA) activity scores, which is a self‐reported questionnaire that evaluates the frequency and type of activity. Patients with scores of 9 or 10 were classified as high‐impact and patients with scores of 1‐4 were classified as low‐impact. Scores of 9‐10 indicate that the subject sometimes or regularly participates in impact sports such as jogging, tennis, skiing, acrobatics, ballet, heavy labor or backpacking. Scores of 1‐4 indicate that the subject is inactive to regularly participating in mild activities such as walking, housework or shopping. These authors found that the high‐impact group had a greater wear rate and increased likelihood of revision surgery within 15 years of surgery.24 The wear rate in the high‐impact group was more than twice that of the rate in the low‐activity group (0.14 mm/yr vs 0.06 mm/yr). Twenty percent of subjects in the high‐impact group underwent revision for mechanical failure (loosening) and 6.5% in the low activity group required revision THA. One interesting point to note is that subjects in the high‐activity group had significantly better self‐reported scores for symptoms, and ability to perform activities of daily living and sports activities. There is potentially some trade‐off between quality of life after THA and the wear rate after surgery.
Advances in implant design
In light of the problems with polyethylene spacer debris and the increasing prevalence of younger patients with hip OA, “hip resurfacing” has become a procedure of choice for many younger and more active patients. In this procedure, only the femoral head and acetabulum are replaced and the femoral neck remains intact. This preserves bone stock should the patient require revision or conversion to THA in the future. This procedure is also reported to carry a reduced dislocation risk because the size of the femoral head component is bigger than traditional THA,19 although there is a greater risk of femoral neck fracture.45 In this procedure there is usually no polyethylene spacer and the two articulating components are metallic. Concerns of systemic metal ions46 from mechanical wear have been reported, although the impact of these ions on patient health is not clear. This becomes a particular issue as these implants are specifically designed for and marketed towards patients anticipating returning to higher levels of activity after surgery.
Resurfacing implants have only become popular and common in the past 10‐15 years, which means there is limited long‐term data on outcomes, particularly as it relates to sporting activity. Preliminary results have shown that hip resurfacing surgery does allow patients to participate in sports post‐operatively. Some data suggests that patients return at a reduced level of activity,47 while other data supports an increase in the frequency and intensity of the sporting activity after hip resurfacing.48 In a sample of 117 patients who underwent hip resurfacing, 87% of patients who participated in sports before surgery were able to return to the activity after surgery, including some higher impact activities such as football (soccer), tennis, jogging and squash.49 At a mean follow‐up time of 30 months there were no incidences of fractures or dislocation. A prospective case series of 40 runners found that 33 of the 40 runners were able to resume running after resurfacing, even at competitive levels, without any cases of osteolysis or loosening at a mean follow‐up of 29 months.50 Girard et al50 (using data from the same subject pool as the aforementioned study) found that 82% of subjects after hip resurfacing were able to resume to high impact sports after surgery. At a mean follow up of 44 months, no patients had osteolysis, implant loosening or required revision surgery. Early results are promising, but longer survival data are needed before definitive evidence‐based recommendations can be made on the safety of high impact sports after hip resurfacing.
An alternative to THA and hip resurfacing is ceramic‐on‐ceramic hip arthroplasty. Compared to traditional THA polyethylene components, ceramic materials are more resistant to mechanical wear and are biologically inert, which make them ideal candidates for individuals who anticipate greater amounts of physical activity after surgery. Chana et al followed 120 patients younger than 55 years old (mean age at time of surgery was 45 years) who underwent ceramic‐on‐ceramic implants for 10 years.51 At the follow‐up there was no detectable wear in the prosthesis and no patient exhibited osteolysis. Although the use of ceramic‐on‐ceramic replacements is promising for the younger population, there is some concern over the toughness of the ceramic materials. These components are more brittle and more likely to fracture and fail in the presence of higher loads.52
MEASURING OUTCOMES
Tracking outcomes in patients participating in higher level activities after THA presents a problem. Often times, research papers and clinical outcomes use the Harris Hip Score as a patient reported functional outcome post THA. This score is usually appropriate to measure change within the individual, particularly when they have a low level of function prior to surgery. However, this tool does have a fairly abrupt ceiling effect that can limit its validity in patients with higher levels of function.53 Recently the High Activity Arthroplasty Score (Appendix 1) has been developed in response to the need to quantify higher level of physical activity and sports participation after joint arthroplasty.54 This is a 4 item self‐report questionnaire in which patients rank their ability to partake in different physical activities, including walking, running, and stair climbing. It also has a question about the individual's overall activity level, which ranges from playing competitive sports to being housebound and non‐ambulatory. Younger individuals after THA who fill out the Harris Hip Score tend to cluster into the highest category whereas the same populations demonstrate a wide distribution of scores for the High Activity Arthroplasty Score.54 In patients after total knee arthroplasty, this measure correlates with other validated self‐report questionnaires, but has a higher ceiling effect than other common outcome measures.55
Implications for Rehabilitation
Many patients, particularly younger individuals, routinely participate in sports before THA or expect to return to these activities after surgery.56 Despite the exceptionally high rate of satisfaction with THA, many patients are unable to return to their prior level of sports as a result of the THA. In a study of 911 patients who underwent THA, 26.4% of patients reported they were unable to return to pre‐operative sports as a result of the surgery.56 The underlying reasons for stopping sporting activity level depend on many factors, including the intensity of the sport, the age of the individual and the reason for undergoing THA. As previously mentioned, fear and anxiety of movement have been cited as barriers to returning to sport.13-15 Joint pain and inability to perform the movement are also commonly listed reasons for not returning to sporting activity.57 Physical therapists should discuss the goals of returning to sport with patients and develop rehabilitation strategies that target the individual's impairments and functional limitations that prevent them from returning to their chosen sport activity.
Although there is little evidence supporting a direct link between risk of injury after THA and sports participation, there are several known risks for dislocation and fracture. Physical therapists working with patients in the post‐operative phase should be aware that most dislocations occur within the first 10 weeks22 and exercises and activities that involve large joint excursions should be avoided. Older women (70+ years old) with osteoporosis58 may be at higher risk for fractures and should likely be discouraged from high‐impact sports after THA. Similarly, patients who underwent THA with a posterior approach in which the capsule and soft tissue was not repaired may also be at greater risk for dislocation.18 Patients with THA who participate in high impact activities should be monitored for signs of wear and loosening. This may include groin pain, feelings of instability or presence of a mass in the groin or pelvis.38,59 These individuals should be informed of the risks of engaging in sporting activities, particular sports that end‐range joint motion or high impact loading.
Dynamic control of the lower extremity muscles is crucial for maintaining stability and normal function during high‐impact sports. Sport‐specific training programs and evidence‐based decision paradigms that reduce injury risk are commonplace for patients after anterior‐cruciate ligament rupture,60 ligamentous reconstruction,61 and shoulder injury.62 These training programs identify and address the impairments and functional limitations that may affect the sports performance or place the individual at a greater risk for re‐injury. Rehabilitation for athletes after THA should consist of the same constructs and should ensure that: 1) range of motion is sufficient to complete the desired task (Figure 3), 2) strength is appropriate to resist external torques and forces during the specific sporting movements (Figure 4), 3) proprioception allows for proper placement of the limb during jumping and landing tasks, as well as activities that may require rapid change in direction of movement, such as skiing or tennis (Figure 5), and 4) muscular activity does not involve excessive co‐contraction and patients demonstrate adequate control of the limb during dynamic activities (Figure 6). Evidence‐based guidelines for rehabilitation in the athletic THA population should be developed and tested to ascertain the safety, feasibility, and effectiveness of these approaches.
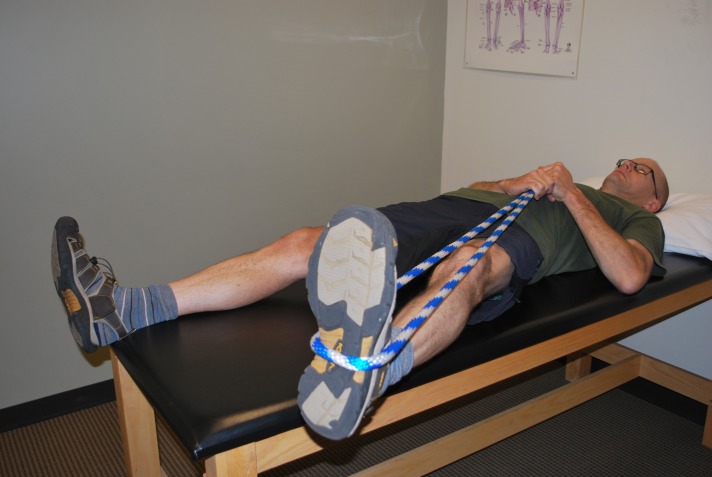
Figure 3.
Self range of motion into abduction using a rope.
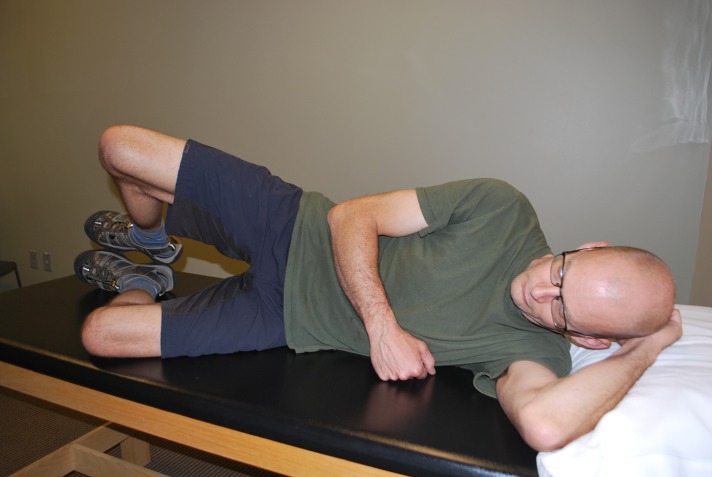
Figure 4.
Clamshell exercise to increase hip abductor/external rotator strength and endurance.
Figure 5.
Landing drills to anticipate proprioceptive demands.
Figure 6.
Limb adapting to changing environment, using single balance on tilt board.
Physical therapists and rehabilitation specialists should also be aware of the patient's physical fitness level before returning to sport is advised. Patients with OA of the lower extremity are often older, more overweight, and at greater risk for cardiovascular events than individuals without joint pain.63 Prior to resuming higher levels of physical exercise, it may be advisable to have the subjects complete a graded exercise test to rule out cardiovascular risk factors.64 The patient's pre‐operative status also needs to be considered during the rehabilitation phase. Therapists should ensure that the patient has had sufficient experience or training in the sporting activity before returning or starting the sport.
CONCLUSIONS
In summary, the risks of returning to high‐level activity after THA include dislocation, periprosthetic fracture, aseptic loosening, and polyethylene/metal wear. There is little prospective evidence regarding the likelihood of poor clinical outcomes with higher level of sporting activity. There is some evidence to suggest that wear may be related to activity level, but the impact on clinical outcomes is conflicting. Future long‐term outcome studies are needed to help determine predictive factors of successfully returning to sports and to better understand potential negative sequelae associated with high‐impact activities after THA. As younger patients are undergoing THA, more individuals are likely to participate in higher‐impact activities. When advising an athlete considering returning to sport after THA, consider their preoperative activity level, current physical fitness, and specific history including bone quality, surgical approach and type of prosthesis.
Acknowledgments
Funding for this study was provided by K12 HD055931: Multicenter Career Development Program for Physical and Occupational Therapy Grant. Both authors contributed equally to this paper.
Appendix 1
High Activity Arthroplasty Score (Max 18 points, higher score is better)
Select your highest level of function in each of the four categories:
1. Walking (max 5 points)
5 Over rough ground > 1 hour
4 Unlimited on flat, rough ground with difficulty
3 Unlimited on flat, no rough ground
2 On flat at least 30 minutes
1 Short distances unassisted (up to 20 minutes)
0 Using walking aids for short distances or worse
2. Running (max 4 points)
4 More than 5 km
3 Jog slowly up to 5 km
2 Run easily across road
1 Run a few steps to avoid traffic if necessary
0 Cannot run
3. Stair Climbing (max 3 points)
3 Climb stairs 2 at a time
2 Climb without handrail
1 Climb with hand rail or stick (cane)
0 Cannot climb stairs
4. Activity Level (max 6 points)
6 Competitive sports e.g. singles tennis, running >10 km, cycling >80 km
5 Social sports e.g. doubles tennis, skiing, jogging <10km, high impact aerobics
4 Vigorous recreation activities e.g. hill‐walking, low impact aerobics, heavy gardening or manual work/farming
3 Moderate recreational activities e.g. golf, light gardening, light working activities
2 Light recreational activities e.g. short walks, lawn bowls
1 Required outdoor activities only e.g. walk short distances to shop
0 Housebound without assistance
Form originally printed in Talbot S, Hooper G, Stokes A, Zordan R. Use of a New High‐Activity Arthroplasty Score to Assess Function of Young Patients With Total Hip or Knee Arthroplasty. Journal of Arthoplasty February 2010, 25(2): 268‐273.
REFERENCES
- 1.Gomez PF Morcuende JA Early attempts at hip arthroplasty‐‐1700s to 1950s. Iowa Orthop J. 2005;25:25‐9. [PMC free article] [PubMed] [Google Scholar]
- 2.Mariconda M Galasso O Costa GG Recano P Cerbasi S Quality of life and functionality after total hip arthroplasty: a long‐term follow‐up study. BMC Musculoskelet Disord. 2011;12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy LB Helmick CG Schwartz TA, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18(11):1372‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz SM Ong KL Lau E Bozic KJ Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624‐30. [DOI] [PubMed] [Google Scholar]
- 5.Ravi B Croxford R Reichmann WM Losina E Katz JN Hawker GA The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637‐47. [DOI] [PubMed] [Google Scholar]
- 6.Clifford PE Mallon WJ Sports after total joint replacement. Clin Sports Med. 2005;24(1):175‐86. [DOI] [PubMed] [Google Scholar]
- 7.Grant E. Breakthrough surgery gives Rangers pitcher, “ultimate stoic” Colby Lewis another chance. Dallas Morning News ‐ Sport DFW. 2014. Available at: http://www.dallasnews.com/sports/texas-rangers/headlines/20140413-breakthrough-surgery-gives-rangers-pitcher-ultimate-stoic-colby-lewis-another-chance.ece.
- 8.Custance C. Ed Jovanovski really is man of steel. ESPN Mag. 2014. Available at: http://espn.go.com/nhl/story/_/id/10367157/nhl-florida-panthers-ed-jovanovski-back-hip-resurfacing-surgery.
- 9.Nightengale B. Bo Jackson reflects on past life 21 years after his All‐Star blast. USA Today. 2010. Available at: http://usatoday30.usatoday.com/sports/baseball/allstar/2010-07-11-bo-jackson-all-star-game-bo-knows_N.htm?csp=34.
- 10.Klein GR Levine BR Hozack WJ et al. Return to athletic activity after total hip arthroplasty. Consensus guidelines based on a survey of the Hip Society and American Association of Hip and Knee Surgeons. J Arthroplasty. 2007;22(2):171‐5. [DOI] [PubMed] [Google Scholar]
- 11.Ritter MA Meding JB Total hip arthroplasty. Can the patient play sports again? Orthopedics. 1987;10(10):1447‐52. [DOI] [PubMed] [Google Scholar]
- 12.Swanson EA Schmalzried TP Dorey FJ Activity recommendations after total hip and knee arthroplasty: a survey of the American Association for Hip and Knee Surgeons. J Arthroplasty. 2009;24(6 Suppl):120‐6. [DOI] [PubMed] [Google Scholar]
- 13.Delasotta LA Rangavajjula A V Porat MD Frank ML Orozco FR Ong AC What are young patients doing after hip reconstruction? J Arthroplasty. 2012;27(8):1518‐1525.e2. [DOI] [PubMed] [Google Scholar]
- 14.Abe H Sakai T Nishii T Takao M Nakamura N Sugano N Jogging after total hip arthroplasty. Am J Sports Med. 2014;42(1):131‐7. [DOI] [PubMed] [Google Scholar]
- 15.Huch K Müller KAC Stürmer T Brenner H Puhl W Günther K‐P Sports activities 5 years after total knee or hip arthroplasty: the Ulm Osteoarthritis Study. Ann Rheum Dis. 2005;64(12):1715‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo RY Morrey BF Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64(9):1295‐306. [PubMed] [Google Scholar]
- 17.Fender D Harper WM Gregg PJ Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register. J Bone Joint Surg Br. 1999;81(4):577‐81. [DOI] [PubMed] [Google Scholar]
- 18.Kwon MS Kuskowski M Mulhall KJ Macaulay W Brown TE Saleh KJ Does surgical approach affect total hip arthroplasty dislocation rates? Clin Orthop Relat Res. 2006;447:34‐8. [DOI] [PubMed] [Google Scholar]
- 19.Kostensalo I Junnila M Virolainen P, et al. Effect of femoral head size on risk of revision for dislocation after total hip arthroplasty: a population‐based analysis of 42,379 primary procedures from the Finnish Arthroplasty Register. Acta Orthop. 2013;84(4):342‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girard J Kern G Migaud H Delaunay C Ramdane N Hamadouche M Primary total hip arthroplasty revision due to dislocation: prospective French multicenter study. Orthop Traumatol Surg Res. 2013;99(5):549‐53. [DOI] [PubMed] [Google Scholar]
- 21.Bozic KJ Kurtz SM Lau E Ong K Vail TP Berry DJ The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128‐33. [DOI] [PubMed] [Google Scholar]
- 22.Leichtle UG Leichtle CI Taslaci F Reize P Wünschel M Dislocation after total hip arthroplasty: risk factors and treatment options. Acta Orthop Traumatol Turc. 2013;47(2):96‐103. [DOI] [PubMed] [Google Scholar]
- 23.Smith T Davies L Ingham C Mann C What activities cause hip dislocationϿ. A review of 100 total hip replacement dislocations*. Adv Physiother. 2012;14(2):55‐60. [Google Scholar]
- 24.Ollivier M Frey S Parratte S Flecher X Argenson J‐N Does impact sport activity influence total hip arthroplasty durability? Clin Orthop Relat Res. 2012;470(11):3060‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindahl H Oden A Garellick G Malchau H The excess mortality due to periprosthetic femur fracture. A study from the Swedish national hip arthroplasty register. Bone. 2007;40(5):1294‐8. [DOI] [PubMed] [Google Scholar]
- 26.McGrory BJ Periprosthetic fracture of the femur after total hip arthroplasty occurring in winter activities: report of two cases. J Surg Orthop Adv. 2004;13(2):119‐23. [PubMed] [Google Scholar]
- 27.Melvin JS Karthikeyan T Cope R Fehring TK Early Failures in Total Hip Arthroplasty ‐ A Changing Paradigm. J Arthroplasty. 2013. [DOI] [PubMed] [Google Scholar]
- 28.Ahnfelt L Herberts P Malchau H Andersson GB Prognosis of total hip replacement. A Swedish multicenter study of 4,664 revisions. Acta Orthop Scand Suppl. 1990;238:1‐26. [PubMed] [Google Scholar]
- 29.Lefevre N Rousseau D Bohu Y Klouche S Herman S Return to judo after joint replacement. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2889‐94. [DOI] [PubMed] [Google Scholar]
- 30.Dubs L Gschwend N Munzinger U Sport after total hip arthroplasty. Arch Orthop Trauma Surg. 1983;101(3):161‐169. [DOI] [PubMed] [Google Scholar]
- 31.Cornell CN Ranawat CS Survivorship analysis of total hip replacements. Results in a series of active patients who were less than fifty‐five years old. J Bone Joint Surg Am. 1986;68(9):1430‐4. [PubMed] [Google Scholar]
- 32.Ulrich SD Seyler TM Bennett D, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32(5):597‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sechriest VF Kyle RF Marek DJ Spates JD Saleh KJ Kuskowski M Activity level in young patients with primary total hip arthroplasty: a 5‐year minimum follow‐up. J Arthroplasty. 2007;22(1):39‐47. [DOI] [PubMed] [Google Scholar]
- 34.Harsha AP Joyce TJ Comparative wear tests of ultra‐high molecular weight polyethylene and cross‐linked polyethylene. Proc Inst Mech Eng H. 2013;227(5):600‐8. [DOI] [PubMed] [Google Scholar]
- 35.Schmalzried TP Shepherd EF Dorey FJ, et al. The John Charnley Award. Wear is a function of use, not time. Clin Orthop Relat Res. 2000;(381):36‐46. [DOI] [PubMed] [Google Scholar]
- 36.Pulido L Restrepo C Parvizi J Late instability following total hip arthroplasty. Clin Med Res. 2007;5(2):139‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orishimo KF Claus AM Sychterz CJ Engh CA Relationship between polyethylene wear and osteolysis in hips with a second‐generation porous‐coated cementless cup after seven years of follow‐up. J Bone Joint Surg Am. 2003;85‐A(6):1095‐9. [DOI] [PubMed] [Google Scholar]
- 38.Nazarian DG Zeni JA Management of a pelvic mass following a worn uncemented total hip arthroplasty. J Arthroplasty. 2012;27(2):323.e17‐20. [DOI] [PubMed] [Google Scholar]
- 39.Sochart DH Porter ML The long‐term results of Charnley low‐friction arthroplasty in young patients who have congenital dislocation, degenerative osteoarthrosis, or rheumatoid arthritis. J Bone Joint Surg Am. 1997;79(11):1599‐617. [DOI] [PubMed] [Google Scholar]
- 40.Kuzyk PRT Saccone M Sprague S Simunovic N Bhandari M Schemitsch EH Cross‐linked versus conventional polyethylene for total hip replacement: a meta‐analysis of randomised controlled trials. J Bone Joint Surg Br. 2011;93(5):593‐600. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y‐H Park J‐W Park J‐S The 27 to 29‐Year Outcomes of the PCA Total Hip Arthroplasty in Patients Younger Than 50 Years Old. J Arthroplasty. 2014. [DOI] [PubMed] [Google Scholar]
- 42.Schmalzried TP Szuszewicz ES Northfiels MR, et al. Quantitative Assessment of Walking Activity after Total Hip or Knee Replacement*. J Bone Jt Surg. 1998;80(1):54‐9. [PubMed] [Google Scholar]
- 43.Van den Bogert AJ Read L Nigg BM An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc. 1999;31(1):131‐42. [DOI] [PubMed] [Google Scholar]
- 44.Gschwend N Frei T Morscher E Nigg B Loehr J Alpine and cross‐country skiing after total hip replacement: 2 cohorts of 50 patients each, one active, the other inactive in skiing, followed for 5‐10 years. Acta Orthop Scand. 2000;71(3):243‐9. [DOI] [PubMed] [Google Scholar]
- 45.Shimmin AJ Back D Femoral neck fractures following Birmingham hip resurfacing: a national review of 50 cases. J Bone Joint Surg Br. 2005;87(4):463‐4. [DOI] [PubMed] [Google Scholar]
- 46.Hartmann A Hannemann F Lützner J, et al. Metal ion concentrations in body fluids after implantation of hip replacements with metal‐on‐metal bearing‐‐systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banerjee M Bouillon B Banerjee C, et al. Sports activity after total hip resurfacing. Am J Sports Med. 2010;38(6):1229‐36. [DOI] [PubMed] [Google Scholar]
- 48.Narvani AA Tsiridis E Nwaboku HCI Bajekal RA Sporting activity following Birmingham hip resurfacing. Int J Sports Med. 2006;27(6):505‐7. [DOI] [PubMed] [Google Scholar]
- 49.Fisher NE Killampalli VV Kundra RK Jagodzinski NA Mathur K Reading AD Sporting and physical activity following hip resurfacing. Int Orthop. 2011;35(7):977‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fouilleron N Wavreille G Endjah N Girard J Running activity after hip resurfacing arthroplasty: a prospective study. Am J Sports Med. 2012;40(4):889‐94. [DOI] [PubMed] [Google Scholar]
- 51.Chana R Facek M Tilley S Walter WK Zicat B Walter WL Ceramic‐on‐ceramic bearings in young patients: outcomes and activity levels at minimum ten‐year follow‐up. Bone Joint J. 2013;95‐B(12):1603‐9. [DOI] [PubMed] [Google Scholar]
- 52.Lang JE Whiddon DR Smith EL Salyapongse AK Use of ceramics in total hip replacement. J Surg Orthop Adv. 2008;17(1):51‐7. [PubMed] [Google Scholar]
- 53.Wamper KE Sierevelt IN Poolman RW Bhandari M Haverkamp D The Harris hip score: Do ceiling effects limit its usefulness in orthopedics? Acta Orthop. 2010;81(6):703‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talbot S Hooper G Stokes A Zordan R Use of a new high‐activity arthroplasty score to assess function of young patients with total hip or knee arthroplasty. J Arthroplasty. 2010;25(2):268‐73. [DOI] [PubMed] [Google Scholar]
- 55.Jenny J‐Y Louis P Diesinger Y High Activity Arthroplasty Score has a lower ceiling effect than standard scores after knee arthroplasty. J Arthroplasty. 2014;29(4):719‐21. [DOI] [PubMed] [Google Scholar]
- 56.Wylde V Livesey C Blom AW Restriction in participation in leisure activities after joint replacement: an exploratory study. Age Ageing. 2012;41(2):246‐9. [DOI] [PubMed] [Google Scholar]
- 57.Wylde V Blom A Dieppe P Hewlett S Learmonth I Return to sport after joint replacement. J Bone Joint Surg Br. 2008;90(7):920‐3. [DOI] [PubMed] [Google Scholar]
- 58.Meek RMD Norwood T Smith R Brenkel IJ Howie CR The risk of peri‐prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96‐101. [DOI] [PubMed] [Google Scholar]
- 59.Kwon Y‐M Ostlere SJ McLardy‐Smith P Athanasou NA Gill HS Murray DW “Asymptomatic” pseudotumors after metal‐on‐metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26(4):511‐8. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald GK Axe MJ Snyder‐Mackler L A decision‐making scheme for returning patients to high‐level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8(2):76‐82. [DOI] [PubMed] [Google Scholar]
- 61.Myer GD Paterno M V Ford KR Quatman CE Hewett TE Rehabilitation after anterior cruciate ligament reconstruction: criteria‐based progression through the return‐to‐sport phase. J Orthop Sports Phys Ther. 2006;36(6):385‐402. [DOI] [PubMed] [Google Scholar]
- 62.Wilk KE Meister K Andrews JR Current Concepts in the Rehabilitation of the Overhead Throwing Athlete. Am J Sport Med. 2002;30(1):136‐151. [DOI] [PubMed] [Google Scholar]
- 63.Nüesch E Dieppe P Reichenbach S Williams S Iff S Jüni P All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Myers J Arena R Franklin B, et al. Recommendations for clinical exercise laboratories: a scientific statement from the american heart association. Circulation. 2009;119(24):3144‐61. [DOI] [PubMed] [Google Scholar]