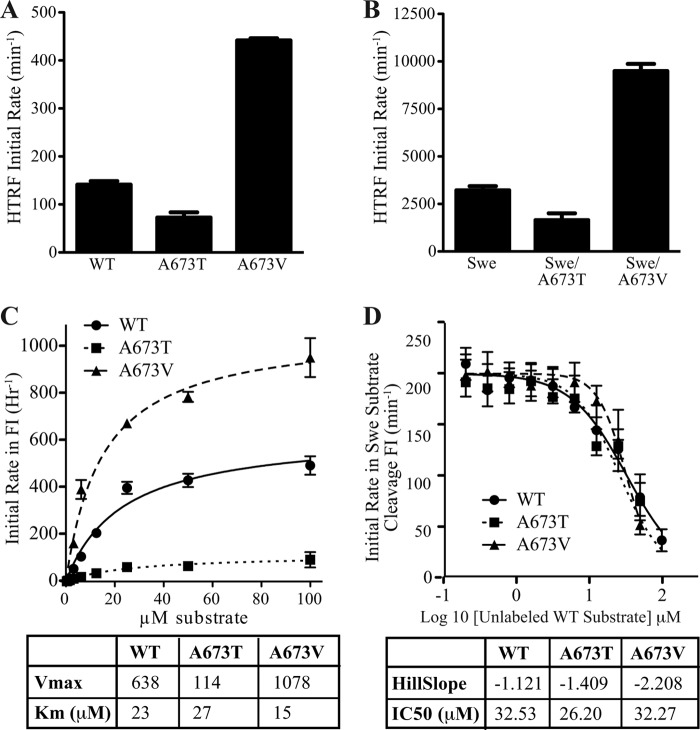
FIGURE 3.
BACE1 processing of APP substrates in a reconstituted in vitro enzyme assay. A and B, TR-FRET analysis of cleavage rates for A673T and A673V peptides in a WT context (A) or in combination with the Swedish (K670N/M671L) mutation (B). C, kinetic analysis of FRET cleavage data. A673T and A673V mutations decreased and increased the rate of catalytic cleavage (Vmax) of the short APP substrate, respectively, without affecting the Km value. D, competitive inhibition of the catalytically efficient Swedish peptide substrate by peptide substrates without Swedish mutation, having a slow cleavage rate. WT, A637T and A673V competitive peptides yielded comparable IC50, suggesting similar binding affinity. Values represent mean ± S.D. of three independent experiments, each with technical replicates.