FIGURE 1.
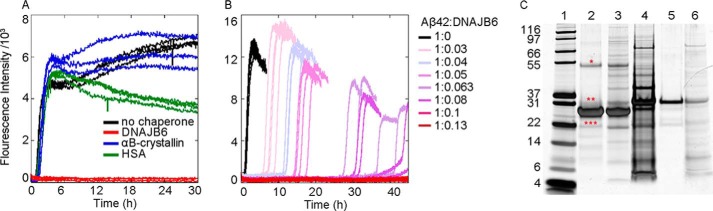
Aβ42 aggregation in the absence and presence of the human molecular chaperone DNAJB6. A, fibril formation by 3 μm Aβ42 solutions was monitored by following the increase in ThT fluorescence (black); the inhibitory effect of DNAJB6 (red) was compared with that of human αB-crystallin (blue) and HAS (green). Molar ratios between Aβ42 and the proteins were 1:0.1. B, aggregation reaction profiles of ThT fluorescence of Aβ42 in the absence (black) and in the presence of DNAJB6 at molar ratios of peptide to chaperone from 1:0.01 to 1:0.13, color coded as indicated to the right. 4 independent incubations per sample were performed. C, silver-stained SDS-PAGE shows the purity of the chaperone with and without the washing step with 8 m urea to remove proteins strongly bound to DNAJB6: lane 1, molecular weight marker (kDa); lane 2, urea-washed DNAJB6; lane 3, not urea-washed DNAJB6; lanes 4 and 5: first and second wash fraction (with and without urea, respectively) resulting in DNAJB6 in lane 2; lane 6, wash fraction without urea resulting in DNAJB6 in lane 3. The asterisks are positioned above bands indicating minor amounts of DNAJB6 degradation product (***), DNAJB6 monomers with His-tag (**), and DNAJB6 dimers (*).
