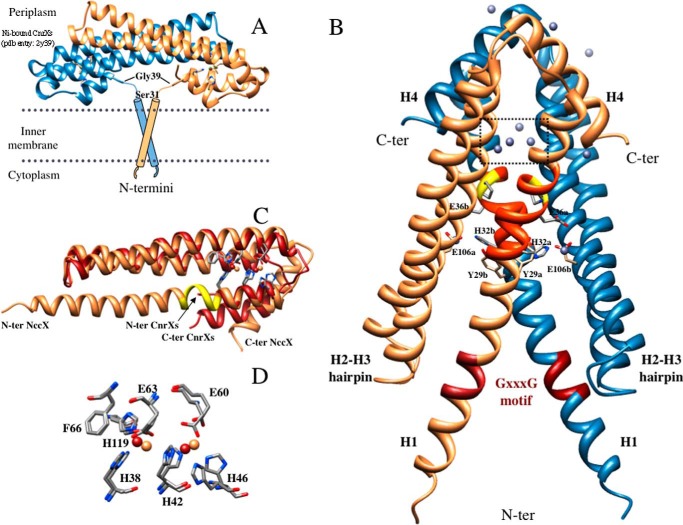
FIGURE 2.
X-ray structure of DPC-solubilized full-length NccX dimer. A, putative model of full-length NccX inserted in the inner membrane adapted from the model of CnrX (16). The two TM segments are supposed to anchor the periplasmic sensor domain. In the CnrXs construct that starts with Ser31, Gly39 was the first N-terminal (N-ter) residue to be structurally defined. B, x-ray structure of NccX determined in this study (Protein Data Bank code 4clv). Protomer A is colored sandy brown, and protomer B is colored steel blue. The side chains of some amino acids stressed in the text are shown as sticks. These amino acids are identified by their one-letter code, numbering in the sequence, and a lowercase letter corresponding to the protomer they belong to. The elements of secondary structure mentioned in the text are annotated (e.g. H2-H3 hairpin) and the location of the zinc ions is shown. The red portion in each H1 helix corresponds to the GXXXG motif. The orange portion corresponds to a stretch of about 10 residues (amino acids 31–40) that were present in the sequence of CnrXs (A) but not structurally defined (16, 19). This stretch includes Pro37 depicted as sticks and highlighted in yellow. Ten zinc ions were located in the asymmetric unit (gray spheres). Most of them have an incomplete coordination sphere at this resolution. The four zinc ions in the dotted square are those of the metal-binding sites detailed in C and D. The ligands of two zinc ions involved in interprotomer interactions are developed as sticks: these are H1 residues Glu36 and His32 contributing one oxygen and one nitrogen, respectively, and Glu106 from H3 of the opposite protomer that contributes one oxygen. C, superimposition of NccX and ZnA-CnrXs protomers. NccX is colored sandy brown, and ZnA-CnrXs is red. The portion of H1 in NccX colored yellow corresponds to the kink upstream at Pro37 that makes a break in the helix as mentioned in B. This kink marks the putative junction between the sensor domain and the TM segment (see “Discussion”). Note that the C-terminal (C-ter) helix H4 of NccX sticks out of the helix bundle. D, close-up view of the superimposition of the metal-binding sites (color code for the zinc ions as for proteins in C). Phe66 is not a metal ligand, but it interacts by π-π stacking with His119, thus providing an interaction between H2 and H3.