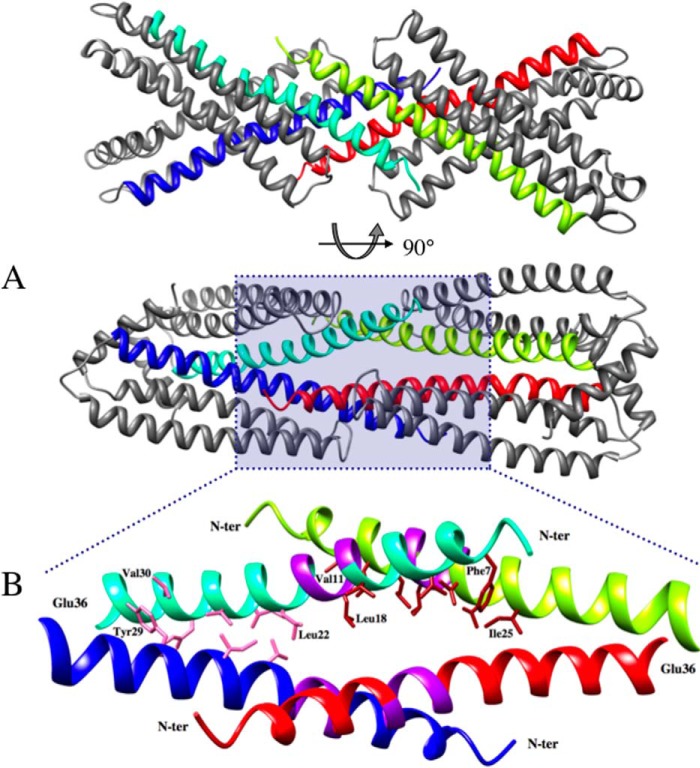
FIGURE 3.
Crystal contacts involving the TM domains of two symmetry-related dimers of NccX. A, the TM domain of dimeric NccX is composed of the N-terminal half of both H1 helices (blue and cyan or red and yellow). The two H1 helices of a TM domain join together at Tyr29, and segments upstream (toward the N terminus) form a fork with an angle of about 55°. This angle is the result of crystal contacts involving two TM domains that are interlocked and run antiparallel to each other (cyan and yellow or blue and red, respectively) (lower panel). B, ribbon representation of residues 4–36 of two interlocked dimers corresponding to a close-up view of the area shaded in blue in the lower panel of A. The N-terminal and C-terminal halves of each TM domain engage in different types of helix-helix interactions. The N-terminal (N-ter) halves of TM helices down to the GXXXG motif form antiparallel dimers with their counterparts from symmetry-related NccX proteins (cyan and yellow or blue and red). The C-terminal halves of TM helices (downstream the GXXXG motif) form parallel dimers within NccX dimers. The color code is as follows: purple, GXXXG motifs; pink, atom contacts between helices with parallel docking within the NccX dimer; brick red, atom contacts between helices with antiparallel docking between interlocked NccX dimers. Some residues making the interface boundaries are indicated.