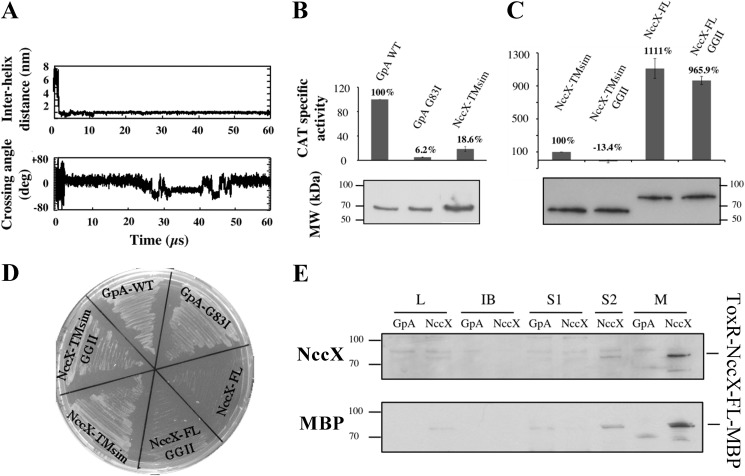
FIGURE 7.
NccX transmembrane segment homodimerization revealed by CG-MD simulations and the TOXCAT assay. A, upper panel, time course of the interhelix distance over a typical 60-μs simulation. Lower panel, time course of the helix crossing angle (Ω) over the same simulation. Positive and negative crossing angles correspond to left-handed and right-handed packings, respectively. B, the ability of NccX TM helices (NccX-TMsim) to homodimerize was assessed with the TOXCAT reporter assay: GpA-WT and GpA-G83I were used as positive and negative controls, respectively. For each construct, CAT specific activity was normalized against GpA-WT, averaged over three independent experiments, and corrected for the production levels of the chimeric proteins ToxR-GpA-MBP and ToxR-NccX-TMsim-MBP that were assessed by Western blot with anti-MBP antibodies (lower panel). C, NccX-TMsim, taken as a reference, was compared with NccX-FL and with the double GGII mutants of either construct. Normalization of the CAT activities was that same as in B. D and E are controls of topology of the constructs used for the TOXCAT assays. D, MalE complementation assays confirmed the correct topology of all constructs but the full-length proteins. For the latter, the bulky metal sensor part of NccX likely hampered the ability of MBP to interact with the maltose transporter. E, the topology of both NccX-FL constructs was found to be correct as they were specifically detected in the membrane fraction. High CAT activity (C) demonstrated the correct orientation of the chimeric protein with ToxR in the cytoplasm. Total proteins were separated by SDS-PAGE and blotted onto a PVDF membrane. ToxR-NccX-FL-MBP was revealed by Western blot analysis with antibodies to CnrXs (upper panel) that cross-react with NccX (10) or with antibodies to MBP (lower panel). The ToxR-GpA-MBP construct was used as a negative control. A very faint band corresponding to ToxR-NccX-FL-MBP was visible in lanes L and S2, whereas this protein was highly detected in lane M corresponding to the membrane fraction. L, lysate obtained by sonic oscillation; IB, inclusion bodies; S1, supernatant of the first centrifugation used to pellet the membrane. The pellet was suspended in sonication buffer and centrifuged again. The new supernatant (S2) and the membrane fraction (M) were used for further analysis. deg, degrees. Error bars in B and C represent S.D.