FIGURE 8.
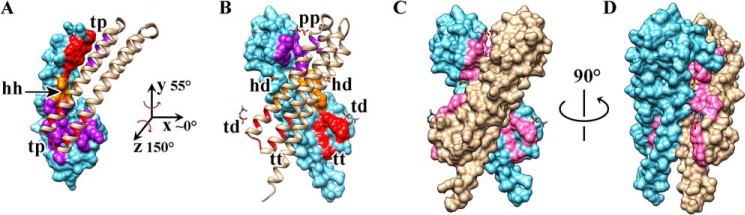
Redistribution of the residues contributing to the dimer interface of the NccX periplasmic domain upon DPC solubilization. A homology model of NccXs, the periplasmic domain of NccX, was built with Modeler (35) using the crystal structure of zinc-bound CnrXs as a template (Protein Data Bank code 2y3d). Chains A (tan) of the NccXs model and NccX crystal structure have been superimposed and are presented side by side as ribbons in A and in B, respectively. Chains B are shown as molecular surfaces (blue). The dimer interface of each protomer of NccXs was considered as a collection of three pieces: the tip of the H2-H3 hairpin (t; red), the body of helix H3 (h; orange), and the platform built by H3-H4 (p; purple). These pieces (t, h, and p) are colored on the molecular surface representation of protomer B of NccXs (A) or NccX (B). The phosphate moiety of some DPC molecules bound to the protein is depicted as orange sticks. Full polar heads of DPC are shown as orange (phosphate moiety) and blue (choline moiety) sticks. A, in NccXs, two protomers stick together via tip-to-platform contacts (tp) and helix-to-helix contacts (hh). B, in NccX, tip-to-platform contacts have been replaced by tip-to-DPC (td), tip-to-TM (tt), and platform-to-platform (pp) contacts. The helix-to-helix contacts have also been disrupted and replaced by helix-to-DPC (hd) contacts. As a result, the dimer interface in crystallized NccX has been completely remodeled as compared with that of NccXs with DPC and the TM N-terminal half sticking in (see text). Rotations about the y and z axes make NccXs chain B superimposable on NccX chain B. C, molecular surface representation of NccX with DPC moieties as sticks. The orientation of NccX is the same as in B. The residues colored in pink are those of the NccXs dimer interface. As a result, regions that were at least partially buried in NccXs and that are exposed to the solvent in NccX do appear in pink on a blue or tan background. D, the same representation as in C was rotated 90° as indicated. DPC moieties co-localized with regions in pink, thus emphasizing the role of DPC in the stabilization of the topology seen in the NccX crystal structure, which is at odds with the topology of the soluble periplasmic domain of NccX, as inferred by homology with CnrXs (10).
